The aim of the present study was to optimizing the protective medium for preservation the viability of S. thermophilus strains after freeze-drying process. The optimal composition of the protective agents was determined according to the selected design of experiments type HA4.Experimental data processing allowed to obtain the regression equation, which describes in natural values (p <0.05) the changes of S. thermophilus viability depending on the content of protective agents in the protective medium. The detailed analysis of the regression equation gives a possibility to conclude that saccharose and sodium citrate mostly contribute and significant increase the viability of S. thermophilus strains after freeze-drying, that also means the keeping important biotechnological properties of studied bacterial strains.
Keywords: Streptococcus thermophilus, freeze-drying, protective medium, optimization
Целью настоящего исследования была оптимизация защитной среды для сохранения жизнеспособности штаммов S. thermophilus после лиофилизации. Оптимальный состав защитных агентов определялся в соответствии с выбранной схемой эксперимента типа HA4. Обработка экспериментальных данных позволила получить уравнение регрессии, которое описывает в естественных значениях (р <0,05) изменения жизнеспособности штаммов S. thermophilus в зависимости от содержания защитных агентов в защитной среде. Детальный анализ уравнения регрессии дал возможность сделать вывод о том, что сахароза и цитрат натрия в большей степени способствуют и значительно увеличивают жизнеспособность S. thermophilus после лиофилизации, что также означает сохранение важных биотехнологических свойств изученных бактериальных штаммов.
Ключевые слова: Streptococcus thermophilus, лиофилизация, защитная среда, оптимизация
The industrial use of lactic acid bacteria (LAB), as biotechnological agents for dairy products, requires their preservation, especially maintaining viability, genetic stability, purity and their biotechnological properties.
Generally, the technology of bacterial concentrates production includes main operations such as preparing and sterilizing the nutrient medium, inoculating by selected strains and accumulation of culture biomass, separating biomass from the culture liquid, transferring the bacterial concentrate into the protective medium, freeze-drying, packing and storing the dried concentrate.
Freeze-drying is a process in which water is frozen, followed by its removal from the sample, initially by sublimation (primary drying) and then by desorption (secondary drying). The main principle involved in freeze drying is a phenomenon, where water passes directly from ice state to the vapor state without passing through the liquid state [1].
An important role in maintaining of the viability of microorganisms during freeze-drying plays the protective medium. As a rule, the protective medium contains lipoprotector agents that preserve microorganisms from the harmful effects of freezing. Their use reduces or prevents the formation of intracellular ice crystals [2].
There are many substances with lyoprotective properties, the mechanism of action of which is of two types: penetrating (penetrating into the cell); non-penetrating (do not penetrate the cell). Penetrating lyoprotectors inhibit the formation of ice crystals due to the formation of hydrogen bonds with water molecules. The most commonly used are: glycerol, propylene glycol, ethylene glycol, dimethyl sulfoxide. The operating principle of non-penetrant lyoprotectors is not yet fully studied. Possibly, the principle of action is to reduce the rate of growth of crystals and protect cells from osmotic pressure difference. The non-penetrant lyoprotectors divide into two groups of substances: oligosaccharides (saccharose and trehalose) and molecular weight such as ficolalbumin, polyvinylpyrrolidone. The following substances are also available: maltodextrin, sorbitol, calcium ascorbate, glutamate [3].
Usually, a protective medium for preserving LAB makes up on a milk-based phosphate buffer with the addition of glycerol, lactose/saccharose, sodium citrate and gelatin [4].
The mechanism for optimizing protection medium was established by developing appropriate mathematical model for calculating the viability index of bacteria considering all possible interactions between the protective substances used.
In this study the optimal composition of the protective agnts for S. thermophilus strains was determined according to the selected design of experiments type HA4 displayed in Table 1. The twenty-four combinations of protective medium of five design factors were run in triplicate.
The mathematical planning of experiments assume the specification of the form of the matrix-system of experiments, consisting of structured data, in which all possible combinations between the levels of influence factors are reflected. These data define the matrix of experiences or, in other words, the system-matrix of planned experiments [5].
The practice of planned experiments, influence factors are attributed to two variance levels: xh a higher level and xl a lower level. These two levels are chosen at a distance equal to the center x0 of the influence factor, called the base level or the zero point, which indicates the value of the influence factors around which the experimental modeling is to be performed. The interval limited by the lower and upper values of the influence factors defines the experimental field. All factors of influence may take values within this range of variation. To simplify the presentation and to generalize the system matrix of the factorial experiments, a coordinate transformation is applied by adopting the following convention: the symbol "+1" is attached to the upper level of the influence factor, the lower level is the symbol "-1", and of the central point (in case of the experiment at three levels of investigation), respectively the symbol "0" [5].
The values of the experimental factors were chosen in a base of preliminary experimental results. The impact of glycerol, saccharose, sodium citrate and gelatin on viability of S. thermophilus strains were studied after freeze-drying.
Cultivation of S. thermophilus strains were performed at optimal parameters in sterilized hydrolyzed milk medium for 6 h at the 40±1 °C till pH 4.6±0.1 was reached. Further, the biomass was separated from the culture liquid by centrifugation at 11000×g for 30±2 min. The biomass was transferred to the protective medium in a ratio 1:1 and then freeze-dried in a lyophilizer (LABCONCO, USA). The freeze-drying process was performing in vacuum and lasted 20±2 h. Under these conditions, bacteria were kept in an anabolic state with limited metabolism.
Enumeration of S. thermophilus was performed using the spread count method. Obtained values were compared with the data before drying. Data were expressed as mean ± standard error using one-way ANOVA by SPSS® version 17.0. Means were compared using Duncan’s multiple range tests, and statistical significance was standard by ANOVA at p < 0.05.
Table 1
Experimental design
|
Sample |
Encoded variables |
Uncoded variables,% (rest of skim milk) |
Viability Y% |
||||||
|
x1 |
x2 |
x3 |
x4 |
x1 |
x2 |
x3 |
x4 |
||
|
glycerin |
saccharose |
sodium citrat |
gelatin |
||||||
|
1 |
+1 |
+1 |
+1 |
+1 |
30 |
15 |
10 |
10 |
84* |
|
2 |
-1 |
-1 |
+1 |
+1 |
10 |
5 |
10 |
10 |
61 |
|
3 |
+1 |
-1 |
-1 |
-1 |
30 |
5 |
5 |
2 |
57 |
|
4 |
-1 |
+1 |
-1 |
+1 |
10 |
15 |
5 |
10 |
90 |
|
5 |
+1 |
-1 |
-1 |
+1 |
30 |
5 |
5 |
10 |
62 |
|
6 |
+1 |
+1 |
+1 |
-1 |
30 |
15 |
10 |
2 |
72 |
|
7 |
-1 |
-1 |
+1 |
-1 |
10 |
5 |
10 |
2 |
58 |
|
8 |
-1 |
+1 |
+1 |
+1 |
10 |
15 |
10 |
10 |
70 |
|
9 |
+1 |
-1 |
+1 |
+1 |
30 |
5 |
10 |
10 |
61 |
|
10 |
+1 |
+1 |
-1 |
-1 |
30 |
15 |
5 |
2 |
62 |
|
11 |
-1 |
-1 |
-1 |
-1 |
10 |
5 |
5 |
2 |
59 |
|
12 |
-1 |
+1 |
+1 |
-1 |
10 |
15 |
10 |
2 |
87 |
|
13 |
+1 |
-1 |
+1 |
-1 |
30 |
5 |
10 |
2 |
52 |
|
14 |
+1 |
+1 |
-1 |
+1 |
30 |
15 |
5 |
10 |
71 |
|
15 |
-1 |
-1 |
-1 |
+1 |
10 |
5 |
5 |
10 |
60 |
|
16 |
+1 |
0 |
0 |
0 |
30 |
10 |
7,5 |
5 |
73 |
|
17 |
-1 |
0 |
0 |
0 |
10 |
10 |
7,5 |
5 |
75 |
|
18 |
0 |
+1 |
0 |
0 |
20 |
15 |
7,5 |
5 |
95 |
|
19 |
0 |
-1 |
0 |
0 |
20 |
5 |
7,5 |
5 |
62 |
|
20 |
0 |
0 |
+1 |
0 |
20 |
10 |
10 |
5 |
97 |
|
21 |
0 |
0 |
-1 |
0 |
20 |
10 |
5 |
5 |
87 |
|
22 |
0 |
0 |
0 |
+1 |
20 |
10 |
7,5 |
10 |
85 |
|
23 |
0 |
0 |
0 |
-1 |
20 |
10 |
7,5 |
2 |
88 |
|
24 |
0 |
0 |
0 |
0 |
20 |
10 |
7,5 |
5 |
89 |
In the result of processing the experimental data was obtained the regression equation (1) which describes in natural values (p <0.05) the changes of S. thermophilus viability depending on the content of protective agents in the protective medium.
Y=56.83–0.25Gl+2.06Z+0.17CS+0.02G (1)
where Y is viability of LAB (%), Gl is glycerol content (%), Z is saccharose content (%), CS is sodium citrate content (%), G is gelatin content (%).
The values and signs of the regression coefficients in equation (1) conclude that saccharose, gelatin and sodium citrate positive influence the viability of freeze-dried strains of S. thermophilus while the effect of glycerol has a negative impact since a high concentration of glycerol has a toxic effect. The main role of saccharose consists in the reduction of the speed of formation of the gelatin crystals during freezing.
A typical 3D surface plot, showing the effect of saccharose, glycerin, and sodium citrate is present in Fig. 1.
A 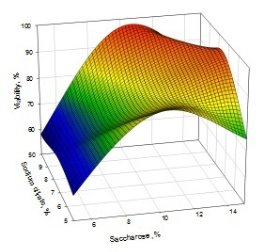 B
B 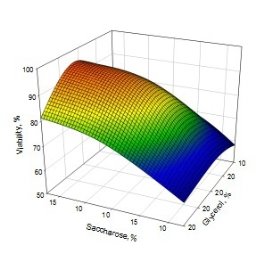
Fig. 1. 3D surface plot displaying the influence of A) sodium citrate and saccharose, B) saccharose and glycerol on viability of freeze-dried S. thermophilus strains
The detailed analysis of equation (1) gives a possibility to conclude that saccharose and sodium citrate mostly contribute and significant increase the viability of S. thermophilus strains after freeze-drying, that also means the keeping important biotechnological properties.
The practical application of the obtained mathematical model (equation 1) will allow determining optimal amounts of protective agents for the production of the starter cultures in the stated range of the sucrose, glycerol and sodium citrate content in the protective medium.
References:
1. Семенов Г. В. Вакуумная сублимационная сушка. — 2013: Москва:ДеЛи плюс, 2013. — 264 с.
2. Arai S. Global view on functional foods: Asian perspectives // British Journal of Nutrition. — 2002. — № 88. — P. 139–143.
3. Uriot O., Denis S., Junjua M., Roussel Y., Dary-Mourot A., Blanquet-Diot S. Streptococcus thermophilus: From yogurt starter to a new promising probiotic candidate? // Journal of Functional Foods. — 2017. — № 37. — P. 74–89.
4. Day J. G., Stacey G. N. Cryopreservation and Freeze-Drying Protocols. — Second Edition. — 2007: Springer Science & Business Media, 2007. — 347 p.
5. Cicala E. F. Metode de prelucrare statistică a datelor experimentale. — 1999: Timişoara: Editura Politehnica, 1999. — 197 p.







