The article examines breakthrough technologies: crypto labels technology and blockchain technology for pharmaceutical industry.
Keywords: blockchain, counterfeit, cryptographic, labels, reader, technology, pharmaceutical industry, ERP-system.
The QuintilesIMS Institute predicts that the pharmaceutical market will reach nearly USD $1.5 trillion by 2021. U.S. market growth will slow by half in 2016 to 6–7 % from 12 % in 2015, and is forecast to average 6–9 % through 2021 [3]. The number of new medicines reaching patients will be historically large with 2,240 drugs in the late-stage pipeline and an expected 45 new active substances (NAS) forecast to be launched on average per year through 2021 [3]. A worldwide increase of counterfeit medicine sales by 90 % over five years was estimated by The World Health Organisation (WHO), with a worldwide sale estimate of $75 Billion in 2010 [5]. Counterfeit medicines pose a significant danger to public health in developing as well as developed countries. Counterfeit medicines may be distributed through different channels such as government and private hospitals, pharmacies or other legitimate or illegitimate distributors. Licensed distributors, pharmacists, health care providers or patients may be unable to detect or differentiate between counterfeit and genuine medicines. It has been difficult to assess the extent of the problem of counterfeit medicines in many settings because of the lack of resources/skills to detect counterfeit medicines, the absence or weak medicines regulatory systems, the different definitions of counterfeit medicines in different countries worldwide, as well as the variations in the distribution systems [4].
Main part
Several countries are now mandating that companies confirm the authenticity of their product by creating a «pedigree» that vouches for a medication's origin and how it has subsequently been handled. The U. S. FDA (United States Food and Drug Administration) has recommended that pharmaceutical companies start using radio frequency identification technology (RFID) as a means of better tracking drugs. Several pharmaceutical companies are experimenting with RFID and optically variable devices (OVDs) or at least using bar codes or other technologies such as web portals that can help track and authenticate the drugs. Some companies are also testing holograms, QR-code (Quick Response Code), color-shifting inks and watermarks that can help them authenticate the package and actual pills. Others are experimenting with using inks or dyes and some are already using tamper-resistant packaging tape on some of their products [5].
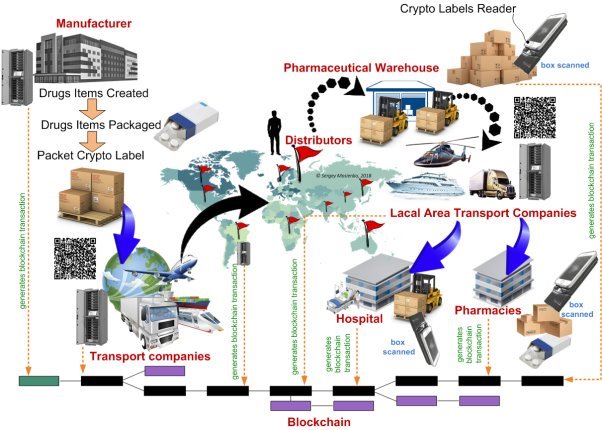
Fig. 1. Crypto labels system and blockchain for drug supply chain
All listed technological solutions (holograms, OVDs, RFID, QR-code) currently being tested are expensive not very effective for protection against counterfeit drugs. A authenticity solution of their drugs based on the holograms, OVDs, bar code, QR-code no future since they do not have the ability to record the “history” or «pedigree» of the passage of drugs from hand to hand. Today people want to know the whole drugs history, from its composition of the treatment that the manufacturer uses, the date of manufacture of the drugs, the time of storage in the warehouses of the distributors, and the time and date of transportation of the drugs by the transporter companies. Today it is not difficult to forge a hologram, OVDs, bar code, QR-code. Нologram’s, OVDs, bar code, QR-code technologies used for labeling drugs can not be connected to new blockchain technology. The chips used for RFID or NFC technology can be cloned without hindrance by hackers. The problem of cloning RFID/NFC chips by hackers has been devoted to a large number of scientific papers. Recommendations U. S. FDA on the use of RFID technology is a mistake.
I want to stop the killing of people from counterfeit drugs. Can this be done today? I think that this can be done with the help of innovative Crypto Labels Systems (CLS) and blockchain technology. The CLS and blockchain solution to this issue works together with the Good Distribution Practice (GDP) regulation adherence previously outlined. The blockchain would enable consumers to track the path of their product throughout the entire supply chain and therefore verify its authenticity. This would be made possible by packages being logged every time they change hands. A crypto labels would be scanned which would enter the transaction onto the immutable blockchain. A product could be therefore scanned at any time by a customer or a producer to view the entire distribution history on the blockchain (see Fig.1).
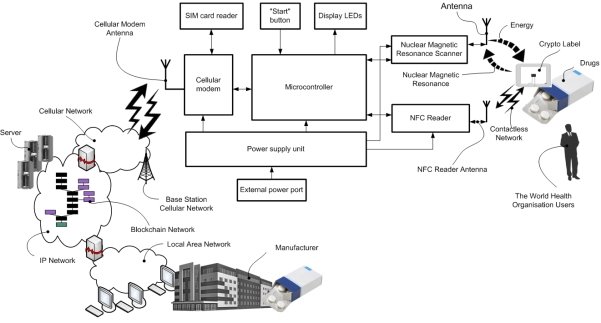
Fig. 2. Crypto labels reader block diagram [1]
It is fully transparent and cannot be changed, and all inputs will only be possible by trusted parties. Another benefit of the system is that if there is disruption in a part of the supply chain, the public ledger of the blockchain provides an efficient and reliable means to track where the issue arose and who was in possession of the shipment at the time. The drug supply chain is a very complicated process involving many transactions, and throughout the entire process adhering to strict regulations is of upmost importance. Currently, manufacturers have little transparency of the supply chain process to track authenticity, which is an issue.
The CLS includes a the Crypto Labels Reader (CLR) with the Cryptographic Labels (CL) and an identification (ID) information system [2] (see Fig. 3).
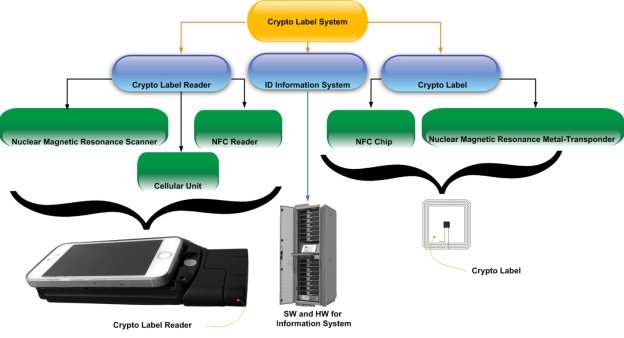
Fig. 3. Crypto label system (technology) composition
The CLR [1] contains a Nuclear Magnetic Resonance (NMR) Scanner and RFID or NFC (Near Field Communication) Reader, cell modem and microcontroller (see Fig.2). The NMR Scanner at the CLR for authenticating and/or identifying of CL for drugs comprise a units generating either continuous or pulse, either modulated or non modulated emitted radiation in the radio frequency band, including a generator of continuous or pulse modulated or non modulated radio frequency signal and an emitting probe head or coil, transforming it into electromagnetic radiation, and a system for detection of the re-radiation emitted by the resonant substance in response to the radio frequency radiation, including receiving probe head or coil and detection device with a registration device determining presence of the re-radiation from the resonant substance.
The СL’s [2] (see Fig.4) consist the RFID/NFC-chip, antenna and insulating material with magnetic resonance metal-transponder (MRMT) on which materials are deposited nuclear magnetic resonance in ferromagnets, or antiferromagnets, or ferrimagnets, or nuclear quadrupole resonance, or very low field electron spin resonance, or said resonance phenomenon is due to electric/magnetic dipole or tunnel transitions between Stark-Zeeman sub levels, or any combinations or aforementioned phenomena. The СL’s, tamper-proof digital fingerprints, to be embedded into products, or devices of products, and linked to the blockchain.
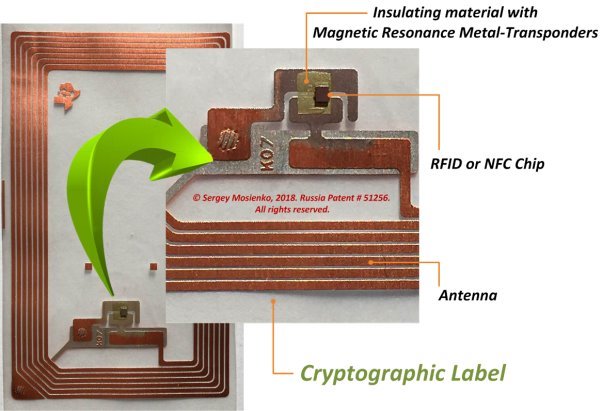
Fig. 4. Crypto label [2]
These fingerprints can take many forms such as tiny NFC-chips, but when they are tied to a blockchain, they represent a powerful means of proving a pharmaceutical industry authenticity. These crypto labels pave the way for new solutions that can combat fraud and protect consumers. The cell modem transmits the information data received from the CL’s via the base stations of the cellular network to the distributed database servers blockchains technology. The blockchain gives internet users the ability to create value and authenticates digital information CL’s. Developing digital identity standards is proving to be a highly complex process. Technical challenges aside, a universal online identity solution requires cooperation between private entities and government. The CLR for cryptographic label system will help solve the blockchains problem. The entire the CLT or the Crypto Labels System (CLS) consists of data carriers (crypto labels, for instance) attached to the drugs (objects) of interests at their manufacturing and distribution centers (plants, storehouses, customs warehouses etc.), crypto labels readers of the aforementioned data carriers (at inspection points, stationary or hand-held) containing cellular modem allowing connection to global network and databases (certain serevr-localized or delocalized — cloud-based). How is it working?
Each drugs of interest should be marked by a certain data carrier — crypto labels. Crypto labels contains information on an drugs, its manufacturing features (origin, batch, time stamp) and destination (where the object should be delivered etc.). The marking occurs at manufacturing facilities, in distribution centers etc. The latter facilities form corresponding data base which stores precise conformity of drugs and data attributed to these drug at marking (see Fig.1). At inspection points crypto labels readers read information from crypto labels and transfer this information to corresponding data bases for checking its validity and providing tracking data. It is very important that information read from crypto labels will not be distorted/counterfeited over all network transactions. Of course, databases themselves must be highly secured. The blockchain technology provides unique uncompromised abilities to protect secure data transfer and processing. Thus, blockchain perfectly protects this part of the crypto labels system. However, all smart blockchain technologies will be absolutely useless in the case when counterfeiters will compromise/fake the data carrier (crypto labels) itself. Let us suppose a counterfeiter will duplicate the adhesive crypto label attached to an authentic drug, and then attach it to the faked drug. Indeed, both crypto label (data carrier) and data base contain full information on authenticity of that drug. The crypro labels reader in the inspection point will read data form an unknown drug and then secure transfer it to data base using advanced blockchain technology. And inform the inspector that the drugs is authentic. Sounds good, doesn’t it? Let us suppose that next time another inspector will read another drug carrying the same data. And then secure transfer it to the data base. Which response will it get from the data base? Is that drug authentic? Data base will inform on duplicated data (the drug was checked before) and put the authenticity of this drug under question. May we call the second drug not authentic (or having other destination etc.)? Which one of these two (three, four etc.) of these drugs was really authentic? Nobody knows!
That is why the blockchain technology only cannot provide real crypto labels system. It is absolutely obvious that the data carrier must be physically protected from duplications/imitations. The crypto labels reader at the inspection point must be confident that the data carrier on an drug is original, i.e. not faked. Thus, the reading device (the CLR) must have some tools for machine recognition of the authenticity of data carrier (the CL's). Nuclear magnetic resonance (NMR) scanner [1] at the crypto labels reader provides perfect tools for such recognition.
Indeed, magnetic resonance metal-transponder (MRMT) provides machine readable invisible tag for physical anti-counterfeiting protection crypto labels (crypto chip) of drugs. Each drug (data carrier) is reliably protected from any unauthorized duplication. For working 10 years in anti-counterfeiting and brand protection business, magnetic resonance metal-transponder having been never compromised, i.e. totally faked or somehow imitated. Magnetic resonance metal-transponder for crypto labels (crypto chip) in combination with blockchain perfectly matches all targets of crypto labels system. In the proposed version of crypto labels system each data carrier it tagged by magnetic resonance metal-transponder for crypto labels.
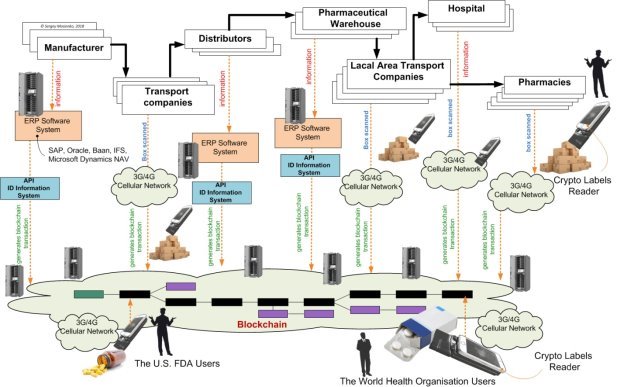
Figure 5. The crypto labels system and blockchain using ERP-system & ID-information system
The crypto labels reader in the inspection point first checks the crypto labels (data carrier) for its authenticity by magnetic resonance metal-transponder for crypto labels. It takes a couple of seconds or even less. In the case the data carrier (crypto labels) is authentic; the crypto labels reader (inspection device) reads data and then securely transfers them (together with the attribute “authentic”) through network to data base. In the case the crypto labels is found to be faked, the crypto labels reader may inform authorities and/or data base on appearance and location of the faked/compromised drug (object). The cell modem transmits the information data received from the CL’s via the base stations of the cellular network to the distributed database servers blockchains technology. The blockchain gives internet users the ability to create value and authenticates digital information crypto labels.
Leveraging blockchain is not about replacing well-established forms of supply chain interactions, such as Enterprise Resource Planning (ERP) software systems (see Fig.4), for example SAP, Oracle, Microsoft Dynamics NAV or IFS.
Rather, as pharmaceuticcal organizations implement new supply chain technologies, for example Internet of Things (IoT) technologies for improved logistics processes monitoring, blockchain will be used provide a synthesized record of information flows. This level of shared visibility will offer drugs plant an opportunity to optimize multi-party supply chain processes drugs.
Conclusions
Developing digital identity standards is proving to be a highly complex process. Technical challenges aside, a universal online identity solution requires cooperation between private entities and government. The crypto labels technology and blockchain technology for pharmaceutical industry is poised as the future of digital transactions, infusing trust, efficiency and transparency into drugs supply chains. Thus, proposed combination of crypto labels technology and blockchains technologies, in sum, creates the only crypto labels system which may be called “secure”.
References:
1. Patent of the RU No.72592 — Modern Identification Wireless Reader.//Mosienko S. A.
2. S. A. Mosienko (2018), Crypto labels technologies integrating blockhain with automotive industry //“Young Scientist”, # 24 (210), June 2018, https://molush.ru/archive/210/51525/
3. The QuintilesIMS Institute Report (2016), Outlook for Global Medicines through_2021,//http://static.correofarmaceutico.com/docs/2016/12/12/qiihi_outlook_for_global_medicines_through_2021.pdf
4. The World Health Organisation (WHO, 2010),// http://www.who.int/medicines/services/expertcommittees/pharmprep/WHO-ACM-3IMPACTSurveyDataCollectionToolReport.pdf
5. The World Health Organisation (WHO, 2010),// https://www.gphf.org/images/downloads/library/who_factsheet275.pdf







