Molybdenum is of very significant biological and physiological importance. It is one of the biological active chemical elements. It plays great role in nitrogen exchange and synthesis of albumen substances, helps in assimilation of dissolved nitrogen, participates in the synthesis of nucleic acids. The amount of hydrocarbons, ascorbic acid, albumen substances, chlorophyll in plants and the intensity of photosynthesis increase by the effect of molybdenum.
Currently the molybdenum is determined by various physical-chemical methods (spectrophotometry, AAS, K-S) in the environmental and industrial objects. The methods to determine the little amount of molybdenum in various natural and biological objects are very expensive and time-wasting. There is almost no ways (test-methods) that is simple, not requiring special equipment for the experiences of ecologists, agro-chemists and agro-technicians to determine the molybdenum in the desert condition.
It is required economically efficient, universal ways having high sensitivity, accuracy and selectivity to determine each chemical element and combinations. The search of new analytical ways or enhancement of analytical opportunities of existing methods is always topical to determine the metal ions on the basis of any method.
Sorption methods of liberation and condensation are widely used in the processing of mineral raw materials and technogenic waste, as well as in analytical practice to determine the necessary sensitivity and selectivity of most determination methods. Sorption analytic systems are considered extremely prospective with the application of chelating polymer sorbents. It is necessary to conduct systematic research to achieve optimal conclusion in each concrete case regardless of most common laws in the literature data on the sorption analytic systems created by using synthetic polymer sorbents.
To study the pollution rate of environment, to evaluate the quality of foods, determination of little amount of metal ions, especially molybdenum in the biological objects are currently topical issues. Having complicated composition of analyzed objects, being very little of the amount of determined micro-component sometimes complicate the analysis, sometimes make impossible to get reliable analysis results. One of the prospective solutions of this problem is elaboration of combined analysis methods in which the initial sorption condensation stage includes. During then application of such methods it is possible to decrease the volume of the sample, to decrease the determination limit, to remove completely or to reduce significantly the effect of the background macro-components and consequently, to increase the repetition and sensitivity of the analysis, to reduce the preparation time of sample for the analysis.
The determination of molybdenum ion in the ultimate permissible condensation limit is considered topical analytical issue in the ecological monitoring of the environmental objects. Being very little of the condensation of this element in most objects and hindering effect of other matrix components do not enable to determine them reliably even with the ultimate physical and physical-chemical methods. Therefore it is necessary to condensate initially this element.
Graduation work has been done according to the scientific works done by the chair of “Ecological chemistry” of the faculty of Ecology and soil-study of Baku State University.
The objective of the work. Doing initial condensation during the determination of the very little amount of molybdenum in various natural and industrial objects such as natural and sewage waters, in soil, in plants, in foods, etc, is one the most important issues of ecological chemistry.
The main objective of the thesis consists of liberation of molybdenum by condensing it with sorbents in environmental objects and elaboration of determination methods characterized by high analytic parameters.
The following issues have been solved to achieve the set objective:
- Studying a number of physical-chemical characters of sorbents; studying desorption and dependence of sorbent on pH, time, ionic force, metal condensation.
- Elaboration of new condensation way and the determination of micro-amount of molybdenum ion in different environmental objects by condensing it upon the obtained practical conclusions.
– Studying selection principles of optimal sorption systems for the liberation, condensation and determination of molybdenum ion in real environmental objects.
Determination of molybdenum (VI) ion in seawater by condensing it with M1 sorbent
1 l is taken from seawater and filtered to remove micro-mixtures. 100 mg M1 sorbent is taken and added to the multi-bottle with the diameter of 0,5 cm. The acidity of filtered analyzed water sample is reached to pH 6–7 by the help of nitrate acid and carried out from mini-bottle in which there is sorbent with the optimal speed (1,2 ml/min). After sorption process 5 ml is washed with 1,0 M HClO4. The amount of molybdenum (VI) ion in the obtained eluate is estimated upon the graphic with set degree. The results of the analysis are given in schedule 1. maximum permissible concentration of molybdenum in seawater is 0,0004 mg/l.
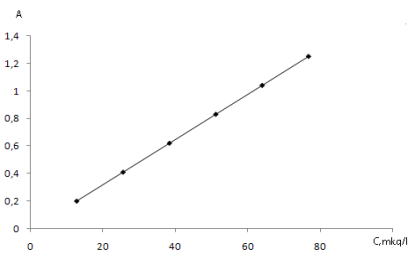
Fig. 1. Degreed graphic of Mo (VI) ion
|
Sample size, l |
Included,mkq/l |
Found, mq/l |
|
1 |
- 5 10 15 |
27,2 ± 0,4 32,3 ± 0,5 37,1 ± 0,6 42,3± 0,8 |
Schedule 1.
The results of the determination of Mo (VI) ion in seawater by condensing it with M1 (the volume of the sample 1000 ml; the volume of eluate 5 ml; msorp=100,000 mg; P=0.95; n=5)
|
Volume of the sample |
Added, mkg/l |
Found, mg/l |
|
1 |
- 5 10 15 |
27,2±0,4 32,3±0,5 37,1±0,6 42,3±0,8 |
*Sample — has been taken from the coats of Nardaran settlement, Caspian Sea.
The determination of molybdenum in artesian water by condensing it M2 sorbent
The samples of artesian water have been taken from different areas of the republic (Gabala, Goychay, Aghdash, Gakh, Balakan, Masalli, Jalilabad, Gazakh, Tovuz, Gusar, Guba, Khudat, Khachmaz) for the research.
1 l is taken from each sample of artesian water separately and filtered for removing the micro-mixtures. 100 mg M2 sorbent is taken and added to the mini-bottle with the diameter of 0,5 cm. The acidity of filtered analyzed artesian water sample is reached to pH 6–7 by the help of nitrate acid and carried out from mini-bottle in which there is sorbent with the optimal speed (1,5 ml/min). After sorption process 5 ml is washed with 2,0 M HClO4. The condensation of molybdenum (VI) ion in the obtained eluate is estimated upon the graphic with set degree. The results have been estimated with the 100 % liberation possibility of the determined metal. The results of the analysis are given in schedule 2. maximum permissible concentration of molybdenum in artesian water is 2,5 mg/l.
Schedule 2
The determination of molybdenum in artesian water by condensing it M2 sorbent (n=5; P=0,95)
|
Area |
Found Mo, mg/l |
|
Gabala |
3,25 |
|
Goychay |
5,50 |
|
Aghdash |
16,8 |
|
Gakh |
7,25 |
|
Balakan |
1,75 |
|
Masalli |
4.75 |
|
Jalilabad |
22,0 |
|
Gazakh |
8,0 |
|
Tovuz |
16,0 |
|
Gusar |
11,5 |
|
Guba |
7,0 |
|
Khudat |
11,0 |
|
Khachmaz |
5,50 |
As shown in the schedule, the amount of molybdenum in the samples of artesian water taken from the area of Jalilabad, Tovuz, Aghdash is more many times than the permissible condensation limit.
The determination of molybdenum ion in spring water by condensing it M1 sorbent
The samples of spring water have been taken from different areas of the republic (Shaki, Zagatala, Gadabay, Astara, Shabran, Gusar, Guba, Siyazan, Shamakhi) for the research.
1 l spring water taken from each area is filtered separately for removing the micro-mixtures. 100 mg M1 sorbent is taken and added to the mini-bottle with the diameter of 0,5 cm. The acidity of filtered analyzed water sample is reached to pH 6–7 by the help of nitrate acid and carried out from mini-bottle in which there is sorbent with the optimal speed (1,5 ml/min). After sorption process 5 ml is washed with 2,0 M HClO4. The condensation of molybdenum (VI) ion in the obtained eluate is estimated upon the graphic with set degree. The results have been estimated with the 100 % liberation possibility of the determined metal. The results of the analysis are given in schedule 3
Schedule 3
The results of the determination of Mo (VI) ion in spring water by condensing it with M1 sorbent (the volume of the sample 1000 ml; the volume of eluate 5 ml; msorp=100,000 mg; P=0.95; n=5)
|
Area |
Found, mg/l |
|
Shaki |
3,25 |
|
Zagatala |
2,15 |
|
Gadabay |
6,0 |
|
Astara |
4,0 |
|
Shabran |
5,0 |
|
Gusar |
12,9 |
|
Guba |
6,5 |
|
Siyazan |
3,0 |
|
Shamakhi |
2,25 |







