Diabetes mellitus (DM) is one of the most common metabolic diseases, and the number of cases has increased worldwide. According to the International
Diabetes Federation (IDF), the number of patients with diabetes will increase from 387 million in 2014 to 592 million in 2035. With a global prevalence of 8.3 %, DM represents a worldwide problem. Diabetic neuropathies are the most prevalent chronic complications of diabetes. This heterogeneous group of conditions affects different parts of the nervous system and presents with diverse clinical manifestations. Chronic sensorimotor DPN is the most common form of DNA major symptom in DN patients is pain arising as a direct consequence of abnormalities in the peripheral somatosensory system in people with diabetes. The symptoms can be present as severe numbness, paresthesia, or hyperesthesia, however, DPN may be asymptomatic in about 50 % of patients 5 and as the DPN progresses, the painful symptoms usually disappear although they have a substantial impact on the quality of life (QoL).
Keywords: Diabetic Peripheral Neuropathy, type 2 diabetes mellitus, quality of life
Сахарный диабет (СД) является одним из наиболее распространенных метаболических заболеваний, и число случаев заболевания во всем мире увеличилось. По данным Международной Федерации диабета (IDF), число пациентов с диабетом увеличится с 387 миллионов в 2014 году до 592 миллионов в 2035 году. С распространенностью в мире 8,3 %, СД представляет собой всемирную проблему. Диабетическая полинейропатия (ДПН) является наиболее распространенным хроническим осложнениям диабета. Эта неоднородная группа состояний влияет на различные части нервной системы и имеет различные клинические проявления. Хронический сенсомоторный ДПН является наиболее распространенной формой ДН. Основным симптомом у пациентов с ДН является боль, возникающая как следствие нарушений периферической соматосенсорной системы у людей с диабетом. Симптомы могут проявляться в виде сильного онемения, парестезии или гиперестезии, однако ДПН может протекать бессимптомно примерно у 50 % пациентов, и по мере прогрессирования ДПН болевые симптомы обычно исчезают, хотя они оказывают существенное влияние на качества жизни (КЖ).
Ключевые слова: Диабетическая полинейропатия, Сахарный диабет тип 2, качества жизни
Diabetic peripheral neuropathy (DPN) is the most prevalent and troublesome complication in patients with diabetes mellitus (DM), causing morbidity with significant impact on the quality of life of the person with diabetes, and can result in early death. Diabetic neuropathy (DN), which may be focal or diffuse, is diagnosed when diabetic patients complain of symptoms and/ or show signs of peripheral nerve dysfunction after the exclusion of other etiologies. Chronic sensorimotor DPN is the most common form of DNA major symptom in DN patients is pain arising as a direct consequence of abnormalities in the peripheral somatosensory system in people with diabetes.
Pathophysiology. Metabolic and vascular factors and impaired nerve repair mechanisms are likely to contribute to the pathogenesis of diabetic neuropathy [1].
Metabolic factors that have been implicated in the pathogenesis of diabetic neuropathy include the following: Advanced glycosylation end — products (AGEs) AGEs are formed by non — enzymatic combination of some of the excess glucose with amino acids in proteins. Advanced glycosylation of essential nerve proteins has been implicated in the pathogenesis of diabetic neuropathy.
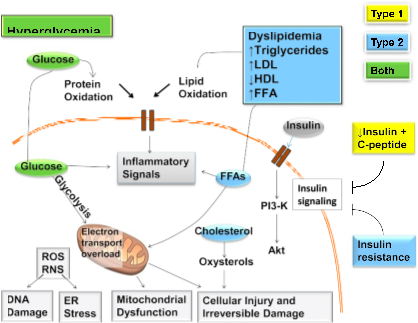
Fig. 1. Mechanisms of diabetic neuropathy. Factors linked to type 1 diabetes (yellow), type 2 diabetes (blue), and both (green) cause DNA damage, endoplasmic reticulum stress, mitochondrial dysfunction, cellular injury, and irreversible damage. The relative importance of the path- ways in this network will vary with cell type, disease profile, and time. ER, endoplasmic reticulum; FFA, free fatty acids; PI3-K, phosphatidylinositol-3 kinase; RNS, reactive nitrogen species; ROS, reactive oxygen species. Adapted and reprinted from Callaghan et al. (20), with permission from Elsevier.
Sorbitol: glucose that enters cells is metabolized in part by the enzyme aldose reductase to sorbitol. This process is more pronounced with chronic hyperglycemia. The accumulation of intracellular sorbitol in tissues such as peripheral nerves results in a rise in cell osmolality, a decrease in intracellular myoinositol and Na — K — ATPase activity, and a slowing of nerve conduction velocities.
Oxidative stress: hyperglycemia results in the accumulation and stabilization of reactive oxygen species, which may damage peripheral nerves.
Vascular risk factors: Morphological abnormalities of the vasa nervorum (small arterioles supplying the nerves) are present early in the course of diabetic polyneuropathy, and parallel the severity of the nerve fiber loss. [2].
Clinical presentations. Diabetic neuropathy may manifest as: distal symmetrical polyneuropathy, polyradiculopathy, mononeuropathy, autonomic neuropathy.
Distal symmetrical polyneuropathy is the most common form of diabetic neuropathy. Patients may present with distal (glove and stocking distribution) sensory loss or paraesthesia, i.e. a sensation of numbness, tingling, burning or sharpness that starts in the feet and spreads proximally. Some patients develop neuropathic pain, typically involving the lower extremities, usually at rest and worst at night. This is occasionally preceded by improvements in glycaemic control. Painful diabetic neuropathy may be acute (lasting less than 12 months) or chronic. Signs of distal symmetrical polyneuropathy include loss of pinprick, temperature, vibration and joint position sensation, and diminished ankle reflexes.
A simple definition of DSPN for clinical practice is the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes. Experimental studies suggest a multifactorial pathogenesis of DSPN (Fig. 1), but the causes remain unknown. A prevailing view of the pathogenesis is that oxidative and inflammatory stress may, in the context of metabolic dysfunction, damage nerve cells. Estimates of the incidence and prevalence of DSPN vary greatly, but evidence from several large observational cohorts and the DCCT/ EDIC suggests that DSPN occurs in at least 20 % of people with type 1 diabetes after 20 years of disease duration. DSPN may be present in at least 10 %–15 % of newly diagnosed patients with type 2 diabetes, with rates increasing to 50 % after 10 years of disease duration. Rates in youth with type 1 and type 2 diabetes approach those observed in adult populations.
DSPN has been associated with glycemia, height (perhaps as a proxy for nerve length), smoking, blood pressure, weight, and lipid measures. There is emerging evidence that DSPN, especially the painful small-fiber neuropathy subtype, may be present in 10 %–30 % of subjects with impaired glucose tolerance, also known as prediabetes or metabolic syndrome. DSPN is the most important cause of foot ulceration, and it is also a prerequisite in the development of Charcot neuroarthropathy (CN). The reader is referred to several other reviews that cover this topic. Foot ulceration and CN are both recognized as late complications of DSPN. These late complications drive amputation risk and economic costs of diabetic neuropathy and are also predictors of mortality. DSPN is also a major contributor to falls and fractures, through more advanced small- and large-fiber dysfunction, with loss of sensory, proprioception, temperature discrimination, and pain, all ultimately leading to unsteadiness, recurrent minor injuries, and an increased risk of falls. These recurrent minor injuries may further contribute to the pathogenesis of CN.
Diabetic polyradiculopathy is characterized by severe pain in the distribution of one or more nerve roots. Diabetic polyradiculopathy usually resolves over 6–12 months. Pain over the chest or abdomen may be due to intercostal or truncal radiculopathy. Sensory symptoms may be accompanied by muscle weakness. Patients with involvement of the lumbar plexus may present with thigh or hip pain and weakness of the hip flexors or extensors (diabetic amyotrophy).
Diabetic patients may present with motor weakness and pain in the distribution of a single cranial or peripheral nerve (e.g. cranial nerves III, IV, VI or VII, median, ulnar or peroneal nerve). Mononeuropathy is less common than polyneuropathy. The most common presentation of mononeuropathy in diabetic patients is ptosis and ophthalmoplegia due to IIIrd cranial nerve palsy. Patients may also present with simultaneous involvement of more than one nerve (mononeuropathy multiplex).
Autonomic neuropathy: Longstanding diabetes may lead to autonomic dysfunction involving the cholinergic, noradrenergic and peptidergic (e.g. substance P, pancreatic polypeptide) systems. Autonomic neuropathy usually involves multiple systems:
Cardiovascular: there may be resting tachycardia, postural hypotension, neuropathic oedema (due to loss of sympathetic vascular innervation and increased peripheral blood flow through arteriovenous shunts).
Gastrointestinal: delayed gastric emptying (gastroparesis) may present with anorexia, nausea, vomiting, early satiety and bloating. Altered small and large bowel motility (autonomic enteropathy) may present with diarrhoea and/or constipation.
Genitourinary: bladder dysfunction (inability to sense a full bladder, failure to void completely, incontinence, recurrent urinary tract infection), erectile dysfunction, retrograde ejaculation and female sexual dysfunction (reduced libido, reduced vaginal lubrication and dyspareunia) can occur.
Hypoglycaemia unawareness arises when reduced adrenaline release results in a loss of the adrenergic symptoms of hypoglycaemia.
Hyperhidrosis (upper extremities), anhidrosis (lower extremities, resulting in dry skin and cracking of the feet and an increased risk of foot ulcers).
Diagnosis. The early recognition and appropriate management of neuropathy in the patient with diabetes is important for a number of reasons:
- Diabetic neuropathy is a diagnosis of exclusion. Nondiabetic neuropathies may be present in patients with diabetes and may be treatable by specific measures.
- A number of treatment options exist for symptomatic diabetic neuropathy.
- Up to 50 % of diabetic peripheral neuropathies may be asymptomatic. If not recognized and if preventive foot care is not implemented, patients are at risk for injuries to their insensate feet.
- Recognition and treatment of autonomic neuropathy may improve symptoms, reduce sequelae, and improve quality of life.
Patients with type 2 diabetes should be assessed annually for DSPN using medical history and simple clinical tests. Up to 50 % of patients may experience symptoms of DSPN (Table 2), whereas the rest are asymptomatic. Patients may not volunteer symptoms but on inquiry may reveal that they are experiencing numbness or other positive symptoms of DSPN. Symptoms vary according to the class of sensory fibers involved. The most common early symptoms are induced by the involvement of small fibers and include pain and dysesthesias (unpleasant sensations of burning). Neuropathic pain may be the first symptom that prompts patients to seek medical care and is present in up to 25 % of individuals with DSPN. Characteristically, the pain is burning, lancinating, tingling, or shooting (electric shock–like); occurs with paresthesias; presents in varying combinations; and is typically worse at night. Neuropathic pain may be accompanied by an exaggerated response to painful stimuli (hyperalgesia) and pain evoked by contact, e.g., with socks, shoes, and bedclothes (allodynia). Neuropathic pain can lead to interference with daily activities, disability, psychosocial impairment, and reduced health-related quality of life. The direct and indirect economic burden associated with neuropathic pain is substantial. The involvement of large fibers may cause numbness, tingling without pain, and loss of protective sensation. Loss of protective sensation indicates the presence of DSPN and is a risk factor for diabetic foot ulceration. Patients can also initially present with an insensate, numb foot due to the loss of large fibers. Patients frequently state that their feet feel like they are wrapped in wool or they are walking on thick socks. It is the loss of the “gift of pain” that permits patients with plantar neuropathic ulcers to walk on the lesions, inducing chronicity, frequently complicated by infection.
Table 1
Symptoms and signs of DSPN
|
|
Large myelinated nerve fibers |
Small myelinated nerve fibers |
|
Function |
Pressure, balance |
Nociception, protective sensation |
|
Symptoms§ |
Numbness, tingling, poor balance |
Pain: burning, electric shocks, stabbing |
|
Examination (clinically diagnostic)** |
Ankle reflexes: reduced/absent Vibration perception: reduced/absent 10-g monofilament: reduced/absent Proprioception: reduced/absent |
Thermal (cold/hot) discrimination: reduced/absent** Pinprick sensation: reduced/absent** |
|
§To document the presence of symptoms for diagnosis; **Documented in symmetrical, distal to proximal pattern. |
||
The following clinical tests may be used to assess small- and large-fiber function distal to proximal (Table 1):
- Small-fiber function: pinprick and temperature sensation
- Large-fiber function: vibration perception, proprioception, 10-g monofilament, and ankle reflexes A 128-Hz tuning fork can be used for the assessment of vibration perception.
Assessment of light-touch perception using a 10-g monofilament should include evaluation on the dorsal aspect of the great toe bilaterally as previously validated by Perkins et al. The 10-g monofilament is a useful clinical tool mainly for detecting more advanced neuropathy and identifying patients at increased risk of ulceration and amputation. Assessments should follow the typical DSPN pattern, starting distally (the dorsal aspect of the hallux) on both sides and move proximally until a sensory threshold is identified. Combining at least two examinations will increase the sensitivity and specificity of detecting DSPN, as demonstrated in several cohorts of patients with type 1 and type 2 diabetes including children and adolescents. The diagnosis of DSPN is principally a clinical one (Table 2). A combination of typical symptomatology and symmetrical distal sensory loss or typical signs in the absence of symptoms in a patient with diabetes is highly suggestive of DSPN and may not require additional evaluation or referral. As up to half of the patients may be asymptomatic, a diagnosis may only be made on examination or, in some cases, when the patient presents with a painless foot ulcer. Clinicians should note that the 10-g monofilament test included for the annual DSPN screening and diagnosis is different than the diagnosis of the “high-risk foot” for ulceration, a late DSPN complication that requires that four sites (first, third, and fifth metatarsal heads and plantar surface of distal hallux) be tested on each foot [3].
The main tests used for the diagnosis of DPN in the studies included in the meta-analysis were the Neuropathy Disability Score (NDS), Neuropathy Symptom Score (NSS), Michigan Neuropathy Screening Instrument (MNSI), 10 g Semmes-Weinstein Monofilament Examination (SWME), and quantitative sensory testing by the vibration perception threshold (VPT), usually combined with clinical assessment and/or electromyography. These tests are useful for evaluating DPN in research and in the clinic, showing similarities and differences between them. The most of diagnosed neuropathies with these tools are related to T2DM and the reason of high prevalence of DPN in adult with T2DM and more ability of these three tools for large fiber neuropathy screening.
QOL is a significant issue in diabetes management, and quick assessment seems prudent for medical screenings in research. Quality of life of the patients was evaluated with SF-36. This health survey questionnaire has an eight-scale profile evaluating physical and mental health. Physical health domain includes physical functioning, role-physical, body pain, and general health. The mental health domain measures vitality, social functioning, role-emotional, and mental health. The scores range from zero, the lowest possible score, to one hundred, with 100 representing the highest level of functioning and representing best health possible. T2D, as an illness, represents a collection of challenges that may affect several different facts of an individual’s functioning, including physical, emotional, social, sexual, cognitive and self-perceptions surrounding health changes. The health issues surrounding T2D are formidable, and a variety of HRQOL instruments have been used throughout the last two decades to gain insight relating to the health perceptions experienced by T2D patients (Luscombe, 2000). Research indicates that obese individuals experience reduced HRQOL, with findings indicating a more significant impact on physical functions relating to health rather than mental capacity (Kolotkin, Crosby, & Williams, 2002), as a meta-analysis of research examining 43,086 study participants indicated that increased BMI status relates to significant reductions in physical QOL, with the highest impact relating to individuals who were class III obese (Ul‐Haq, Mackay, Fenwick, & Pell, 2013). At-risk individuals for T2D include those who are inactive. HRQOL decreased in a linear fashion in relationship to physical inactivity and increased in individuals who had higher physical activity levels. Individuals who were more active reported better subjective health and weight control, while researchers reported that high activity levels reduced the risk of T2D onset and associated complications. HRQOL relating to DPN is an important field of study, allowing for the assessment of disease impact for the potential early intervention and offers unique caveats to physicians to aid them in the diagnosis and treatment of DPN [4].
Medical treatment. In patients with type 2 diabetes with more advanced disease and multiple risk factors and comorbidities, intensive glucose control alone is modestly effective in preventing distal symmetric polyneuropathy and patient-centered goals should be targeted. Lifestyle interventions are recommended for distal symmetric polyneuropathy prevention in patients with prediabetes/metabolic syndrome and type 2 diabetes.
Alpha-lipoic acid. Another therapy that has a disease-modifying effect is alpha-lipoic acid (ALA). Multiple clinical trials have been completed using a variety of study designs, routes of administration, and sample sizes. In the Alpha-Lipoic Acid in Diabetic Neuropathy [ALADIN] III trial, which was a multicenter, double-blind, randomized placebo-controlled study, there was a small but significant improvement in the Neuropathy Impairment Score (NIS) of patients treated with ALA, but no significant improvement in the Total Symptom Score (TSS).
The neuropathy that occurs in patients with impaired glucose regulation is typically characterized by distal, symmetric sensory symptoms including pain. This pattern suggests prominent involvement of small nerve fibers including unmyelinated (C) fibers and thinly myelinated (Aδ) fibers. Pain is frequently the complaint that motivates patients to seek medical care, and it is often difficult to treat. This is a concern because neuropathic pain can have a negative impact on quality of life; it has been reported to interfere with general activity, mood, mobility, work, social relations, sleep, leisure activities, walking ability, and enjoyment of life. Medications used to treat painful diabetic small-fiber neuropathies include anticonvulsants, antidepressants, topical anesthetics, and both narcotic and non-narcotic analgesics [5].
Neuropathic pain can be severe and can impact quality of life, limit mobility, and contribute to depression and social dysfunction. No compelling evidence exists in support of glycemic control or lifestyle management as therapies for neuropathic pain in diabetes or prediabetes, which leaves only pharmaceutical interventions [6].
Pregabalin and duloxetine have received regulatory approval by the FDA, Health Canada, and the European Medicines Agency for the treatment of neuropathic pain in diabetes. The opioid tapentadol has regulatory approval in the U. S. and Canada, but the evidence of its use is weaker. Comparative effectiveness studies and trials that include quality-of-life outcomes are rare, so treatment decisions must consider each patient’s presentation and comorbidities and often follow a trial-and-error approach. Given the range of partially effective treatment options, a tailored and stepwise pharmacologic strategy with careful attention to relative symptom improvement, medication adherence, and medication side effects is recommended to achieve pain reduction and improve quality of life [7].
Pregabalin, a calcium channel a2-d subunit ligand, is the most extensively studied drug for DPN. The majority of studies testing pregabalin have reported favorable effects on the proportion of participants with at least 30–50 % improvement in pain. However, not all trials with pregabalin have been positive, especially when treating patients with advanced refractory DPN. Adverse effects may be more severe in older patients and may be attenuated by lower starting doses and more gradual titration. The related drug, gabapentin, has also shown efficacy for pain control in diabetic neuropathy and may be less expensive, although it is not FDA approved for this indication. Duloxetine is a selective norepinephrine and serotonin reuptake inhibitor. Doses of 60 and 120 mg/day showed efficacy in the treatment of pain associated with DPN in multicenter randomized trials, although some of these had high drop-out rates.
Duloxetine also appeared to improve neuropathy-related quality of life.
In longer-termstudies, a small increase in A1Cwas reported in peoplewith diabetes treated with duloxetine compared with placebo. Adverse events may be more severe in older people but may be attenuated with lower doses and slower titrations of duloxetine.
Tricyclic antidepressants, venlafaxine, carbamazepine, and topical capsaicin, although not approved for the treatment of painful DPN, may be effective and considered for the treatment of painful DPN [8].
References:
- IDF. International Diabetes Federation: IDF.org; 2015. Available from: http://www.idf.org/
- Lecture Notes: Endocrinology and Diabetes. By A. Sam and K. Meeran. Published 2009 by Blackwell Publishing. ISBN 978–1-4051–5345–4.
- Diabetic Neuropathy: A Position Statement by the American Diabetes Association Diabetes Care 2017;40:136–154 | DOI: 10.2337/dc16–2042
- Brown, Jennifer J.. «Neuropathy Detection, Quality of Life Tools & Treatment for Type 2 Diabetes” (2016). Doctor of Philosophy (PhD), dissertation, Human Movement Sciences, Old Dominion University, DOI: 10.25777/q2my-6b79 https://digitalcommons.odu.edu/hms_etds/2
- Microvascular Complications and Foot Care: Standards of Medical Care in Diabetesd 2019 Diabetes Care 2019;42(Suppl. 1):S124–S138 | https://doi.org/10.2337/dc19-S011
- Sadosky A, Schaefer C, Mann R, et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospectivechart reviewand cross-sectional survey. Diabetes Metab Syndr Obes 2013;6:79–92
- Waldfogel JM, Nesbit SA, Dy SM, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: a systematic review. Neurology 2017;88:1958–1967
- Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med 2014;161:639







