As it is known, one of the main causes of environmental problems at the end of unforeseen accidents during technological processes in the oil and gas and petrochemical industries is corrosion of steel process equipment. Therefore, as in other industries, one of the main methods of corrosion protection of steel technological equipment in the oil and gas and petrochemical industries is the use of ecologically effective inhibitors. Synthesis and research of new nitrogen-containing organic compounds that meet all the requirements of ecological safety and which are low-cost in the field of organic and petrochemical synthesis are considered as one of the topical issues of the era.
In this regard, as noted in the literature [1], organic compounds containing high quantity of nitrogen atoms possess effective inhibitory properties. Based on the results of our studies [2–4], it has been proved that in fact, various functional groups and organic compounds with high nitrogen atoms have an anti-corrosive effect.
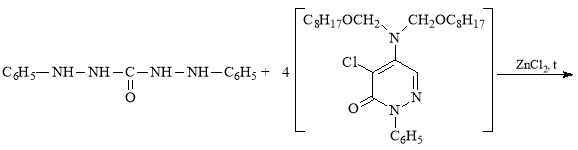
C8H17OCH2Cl (α-chloroctoxymethyl) and N1`N1`-dioctoxymethyl chlorazone ethers [2–3] for the synthesis of the new derivative of diphenylcarbazide (compound B-1) were obtained according the methods described in literature. The composition and structure of these ethers were determined by known methods. The results obtained are in line with the indicators given in the literature [2–3].
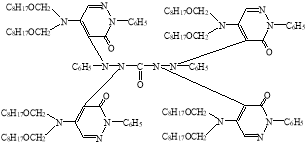
In view of the foregoing, we have synthesized a new derivative of diphenylkarbazide B-1, based on the chlorazone ether of the two-CH2OC8H17 group. Carrying out the reaction of diphenylcarbazide with N1`N1`-dioctoxymethylchlororazone ether according to the procedure described in the literature [2–3], a new, unknown in the literature diphenylcarbazide derivative N1-(N1`N1`-dioctoxymethyl)azone-N2-(N1`N1`-dioctoxymethyl)azone-N4-(N1`N1`-dioctoxymethyl)azone-N5-(N1`N1`-dioctoxymethyl)azone)diphenylcarbazide, compound B-1.
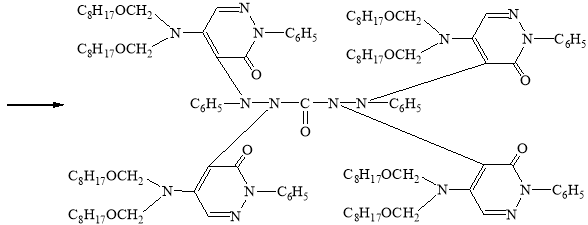
B-1
The output of the synthesized new B-1 compound of the diphenylcarbazide, its the physical and chemical constants, and the element analyzes are given in Table 1.
Research has been carried out to determine the corrosion rate and inhibitory efficacy of this compound in a highly aggressive environment. According to the study, the inhibitory effect of this compound at the lowest 0.5, 1.0, 1.5 mg/l density was 99.96–100 %. Determining the inhibitory efficiency of the new derivative of the synthesized diphenylcarbazide (compound B-1) has been investigated in accordance with method specified in «gravimetric».
The ecological efficiency of the inhibitory property of the new derivative of diphenylcarbazide — compound B-1, can be explained as follows. Based on the results of our previous studies [2–4] and also the research data in the literature [1,5–12] it can be noted that with the formation of 16 nitrogen atoms in the new diphenylcarbazide derivative, 8 CH2OC8H17 groups, 31 double bond set, the metal surface becomes passive due to an increase in the influence of electron density and the internal Van der Waals forces of the compound. As a result, the compound B-1 even at low densities completely reduces the corrosion rate of the metal in the most aggressive environments. Thus, the new derivative B-1 of diphenylcarbazide, shown in Table 2, can be used as an ecologically and economically important inhibitor of corrosion protection of steel technological equipment operated in the most aggressive environments in the oil and gas and petrochemical industries.
Experimental part
Synthesis of N1-(N1`N1`-dioctoxymethyl)azone-N2-(N1`N1`-dioctoxymethyl) azone-N4-(N1`N1`-dioctoxymethyl)azon-N5-(N1`N1`-dioctoxymethyl)azonedi-phenylcarbazide compound (B-1)
The synthesis tube is filled with 2g ZnCl2 catalyst and 0.01 g/mol diphenylcarbazide, and 50 ml of C2H5OH alcohol is added and at 700C is mixed until complete dissolution of diphenylcarbazide. Then, 0.04 g-mol N1',N1' -diocoxymethylchlorazone ether was added to the mixture from a dropping funnel at regular intervals and stirred for 6 hours at 76 °C. After the reaction is complete, 100 ml of 10 % NaOH solution and 100 ml of distilled water are added to the mixture. Then, 25 ml of diethyl ether is added to the reaction mixture and mixed. The mixture is filled into a separation funnel, and after the organic layer is separated from the water layer, diethyl ether in the organic layer is distilled off by a water pump. After being deposited on CaCl2, an organic layer is pumped into vacuum. The composition and structure of the new derivative of diphenylcarbazide (compound B-1) on the basis of N1`N1`-dioctoxymethyl chlorazone ether was determined by IR and magnetic mass spectra.
In the IR spectrum of the synthesized N1-(N1`N1`-dioxtoxymethyl)azone- N2-(N1`N1`-dioctoxymethyl)azone-N4-(N1`N1`-dioctoxymethyl)azone-N5-(N1`N1`-dioctoxymethyl)azonediphenylcarbazide (B-1) compound there are -C-O-C- simple ether group 1050, 1080 cm-1; CH2 group 2950 cm-1; CH3 group 1380, 1460, 2960, 3030 cm-1; C-N bond 1310–1350 cm-1; N-N group 900, 1580 cm-1; C=C bond 1680 cm-1 in the azone group; C = C bonds 1440, 1465, 1500, 1510, 1590–1600 cm-1 in benzene nuclei; C6H5 group 700–780 cm-1.
The molecular mass of that compound in the magnetic mass spectrum corresponds to the molecular ion of 2118 m/e.
Table 1
Data on the composite output, physical and chemical constants, elemental analysis of the synthesized N1-(N1`N1`-dioctoxymethyl)azone-N2(N1`N1`-dioctoxymethyl)azone-N4-(N1`N1`-dioctoximethyl)azone-N5-(N1`N1`-dioctoximethyl)azonediphenylcarbazide compound (B-1)
|
Chemical formula and conditional No. of the compound |
Output% |
Tboil. 0C (760 Torr) |
d420
|
nd20 |
MRDfound / calculated |
Brutto formula, mol.weight |
Elemental analysis%, calculated / found |
||
|
C |
H |
N |
|||||||
|
B-1 |
98.94 |
278–279 (2) |
1.5158 |
1.8627 |
63096/ 630.74 |
C125H186N16O13 2118 |
70.82/ 70.61 |
8.78/ 8.66 |
10.58/ 10.43 |
Table 2
Results of the study of the inhibitory efficiency of anew derivative of diphenylcarbazid (B-1) based on N1`N1`-dioctoxymethylchlorazone ether
|
conditional No. of the compound |
İnhibitor density, mg/l |
3% NaCl (10:1)+H2S 500 mg/l |
0.3N HCl+benzine (1:7)+ H2S 1000 mg/l |
||
|
Corrosion rate g/cm hour |
Inhibitor efficiency,% |
Corrosion rate, g/cm hour |
Inhibitor efficiency,% |
||
|
Without Inhibitors |
- |
2.56 |
- |
3.65 |
- |
|
B-1 |
0.5 1.0 1.5 |
0.0003 0.0002 0.0001 |
99.96 99.99 100 |
0.0002 0.0001 — |
99.99 100 — |
|
A [4] |
200 |
0.038 |
98.5 |
0.078 |
98 |
References:
- Rachev H., Stefanova S. Handbook of corrosion. //M.: Mir, 1982. P.62 (in Russian).
- Bayramov G. I. Synthesis and investigation of newly proposed guanidine on the base of khloralkyl and alkenyl-oxymethyl ethers and chlorazone. //J. Natural and tekhnical tic and Technical Guide, No.2, 2009, p. 37–43 (in Russian).
- Gadzhiyeva S. R. Bayramov G.I, Aliyeva T. I., et al. Synthesis and utilization of novel sulfateimethylene sulfide-based dioxymethylchlorazone ether as an ecologically effective inhibitor// Young scientist. International scien. Journal No. 6, 2019, p.1–5 (in Russian).
- Shikhmamedbekova A. Z., Mamedyarova I. F., Bayramov G. I., Mamedaliyeva G. G., et al. N, N '- biphenyl — N' — octoxytmetyl-guanidine as the corrosion inhibitor of steel in two-phase system. Copyright certificate. USSR, No. 1031141, 1983, А С07 С129 / 12; P23 F 11/14 (in Russian).
- Shel N. V., Tsygankova L. E., Novelty inhibitors of corrosion of metals for oil and petrochemical products. //XVIII Mendeleev's Session on Chemistry and Applied Chemistry. 2007, p. 608 (in Russian).
- Faritov A. T., Khudyakova L. P., et al. Methodology for the selection of corrosion inhibitors for OAO Orenburgneft // Problems of the collection, preparation and transport of oil and oil products: Sat. scientific tr IPTET.- UFA, 2003.- Issue. p. 167–171(in Russian).
- Shirayeva R. N., Kudasheva F. H., Gumayev N., et al. Inhibition of resin deposits of asphaltenes and paraffins in oil pipelines with chemical reagents // J. Chemistry and technology of fuels and oils. M.: Publishing House of the Russian State University of Oil and Gas named after I. M. Gubkin, No. 3, 2009, p.52–53 (in Russian).
- Khudyakova L. P. A system for ensuring the safe operation of oil and gas equipment and pipelines operating in an aggressive manner// SUE. IPTER, Ufa. 2008, p. 39 (in Russian).
- Investigation of the effect of hydrogen sulfide on corrosion processes in the operational characteristics of structural elements of pipelines and tanks. // Problems of the collection, preparation and transport of oil and oil products: Sat. Scientific Engineering, IPTER-2005, m Issue 65. from. 27–40 (in Russian).
- Khudyakova L. P., Spashenko A. Y., Antipov Yu.N. Assessment of the degree of danger of stress-corrosion cracks // STJ «Problems, collection, preparation and transport of oil and oil products», IPTER. Ufa. 2007. Issue 3(69), p. 39–41(in Russian).
- Khudyakova L. P., Spashenko A.Yu. and others. Estimated rate of corrosion-mechanical cracking of oil and gas equipment and pipelines.// STJ «Problems, collection, preparation and transport of oil and oil products» / IPTER.Ufa. 2007. Issue 3 (69), p. 61–63 (in Russian).
- Gurvich L. M., Sherstiyev N. M. Multifunctional surfactant compositions for oil production technological operations. M.: VNIIOENG, 1994, p.268 (in Russian).
- Frolova L. V., Agafankin F. V. Inhibition of hydrogen sulfide corrosion of carbon steels by N-ethanolbutylene amine and its mixtures with tertiary amine// Journal of corrosion: materials, protection. M.: publ. of LLC «Science and Technology». 2010, No. 1, p.15 (in Russian)