The article shows the results of research on the influence on mass ratio СаО: Р2О5 in phosphate raw material on the quantity of different forms of calcium phosphates with neutralization of nitric acid extraction by ammonium humate, and also optimal degree of ammonation (рН=4,5) is stated, when loss of digestible Р2О5 is not big, and hygroscopicity of the product meets the requirements of its storage in Uzbekistan.
As it is known, agricultural production may have very serious problems, one of which is related to soil humus.
The specific role of humus is in his positive influence on all sides of soil fertility.
Humus improves physical features of soil, creates optimal water-air regime, prevents washout and wash-off of mineral fertilizers and thus assists to environment protection from pollution, turns hard-digestible forms of phosphate to easy digestible.
To create deficit-free balance of soil humus and to improve crop capacity of agriculturу, Uzbekistan needs approximately 45 mln tons of organic fertilizers. Currently even with full mobilization of all manure and dung resources, requirement of tillagein organic fertilizers to reproduce soil humus can be met only by 17–20 % [1]. Therefore, engineering of organic mineral fertilizers production technology on the basis oа nitric acid processing of high-carbonate phosphorites of Central Kyzylkums and humic acids is actual.
According to literary information [2], humic acids with calcium ions make sparingly soluble calcium humates, therefore insertion of soluble humate ammonium salts into nitric acid drawing simultaneously with with its ammonation (till pH=4,5) leads to link parts of calcium ions, owing to it, ratio CaO:P2O5 reduces to liquid phase and possibility of unassimilable forms of phosphates forming.
Besides, calcium humates, which form as a result of reaction, are included to the organic nutritious complex.
Thus, on one side, humic acids are used to link calcium, on the other side — nutritious value of reaction products, calcium humates are used to increase the amount of nutritious components.
The main raw material to produce “humosum” is Central Kyzylkums high-carbonized phosphorites with the following composition, %: Р2О5–17,25; СаО-46,30; MgO-1,78; R2O3–2,42; F-1,87; CO2–15,90; CaO:Р2О5–2,71; insoluble residue — 6,5 and ammonium humates, produced from humic carbons of Angren deposit with the following composition, %: moisture — 10,2; ash content — 22,4; humic acids content — 55,4; residual carbon — 12.
Table 1 shows the characteristic of humic acids and their fractions.
Table 1
Сharacteristic of humic acids and their fractions
|
Acids |
Wa,% |
Aa,% |
Cг,% |
Нг,% |
(O+N+S),% |
COOH C:H (α) |
C:H (atomic) |
Functional acid group, mg-eqv/g |
Optimistic density λ=465 mmk |
Coagulation threshold, Mg-eqv/l |
||
|
COOH |
ОН (fenol) |
amount |
||||||||||
|
humic |
9,10 |
11,0 |
65,6 |
3,40 |
30,91 |
2,4 |
1,60 |
3,92 |
2,20 |
6,12 |
1,35 |
1,6 |
|
gumus |
10,32 |
5,85 |
65,31 |
1,81 |
32,88 |
1,40 |
3,0 |
4,20 |
2,20 |
6,40 |
3,0 |
1,2 |
|
Gimatomelanovie |
- |
- |
64,30 |
3,50 |
32,20 |
4,11 |
1,53 |
6,10 |
1,66 |
7,76 |
0,91 |
4,0 |
|
fulvoasid |
- |
- |
46,63 |
4,60 |
48,77 |
5,60 |
0,80 |
4,75 |
5,28 |
10,03 |
0,54 |
0 |
Physicochemical and agrochemical features (content of assimilable P2O5 form) of finished product generally depend on ammonation degree.
Ammonation experiments were held in three-neck flask supplied with mixer and placed into water bath. HNO3 with concentration 56 %, with stеchiometric norm of 102 % on CaO was used.
Nitric acid exhaust composition (NAE): HNO3–0,79 %; H3PO4–8,82 %; CaO-17,54; MgO-0,67 %; P2O5–0,92 %.
Definition of P2O5 (citrate and water-soluble) was held by standard method [2].
Research results are shown in fig. 1.
As shown in fig.1 with the increase of ammonia neutralization of nitric acid exhaust, assimilable form of P2O5 (curve 1) falls, maximum value 97 % is seen with pH=3–4. Further, with the increase of pH up to 8 ratio Р2О5(ass.):Р2О5(gen) reduces till 93 %. Reduction of assimilable form of P2O5 with the increase of neutralization degree is explained by retrogradation P2O5.
Curve b (fig.1) shows change of water-soluble P2O5 with the increase of ammonation degree.
Rate of curve 2 is more sudden, espetially under values of pH=3–5, evidently that it is connected with intense fallout of dibasic calcium phosphate under pH=4, therefore content of monobasic calcium phosphate reduces.
Under pH=7–8 the content of monobasic calcium phosphate reaches 1 %.
Curve 3 shows cange of hygroscopic point of the product depending on ammonation degree of nitric acid exhaust of phosphorites.
Thus, hygroscopic point of the product increases with the increase of ammonation degree.
It evidently can be explained by forming of less hygroscopic products under neutralization, such as dibasic calcium phosphate (hygroscopic point СаНРО4·2Н2О according to N. E. Pestov under temperature of 25°C is equal to 64 %). hygroscopic point for saturated solution of calcium nitrate, one of the main products under nitric acid decomposition of phosphates, under temperature of 25°C is equal to 50,5 %, and of calcium nitrate — 42,5 %.
As a result, optimum degree of ammonation (pH=4,5) was installed, under which loss of assimilable P2O5 is small, and hygroscopicity (absorbability) of the product meets its storage conditions in Uzbekistan.
At optimal degree of NAE ammonation, influence of ammonium humates on the quantity of monocalcium phosphate, dicalcium phosphate, tricalcium phosphate and retrogradation of Р2О5.
Under optimum degree of nitric acid exhaust ammonation, influence of ammonium humates was studied. Norm of ammonium humate is chosen from quick lime content analysis in initial phosphorite. The results of the research are shown on fig.2 and table2.
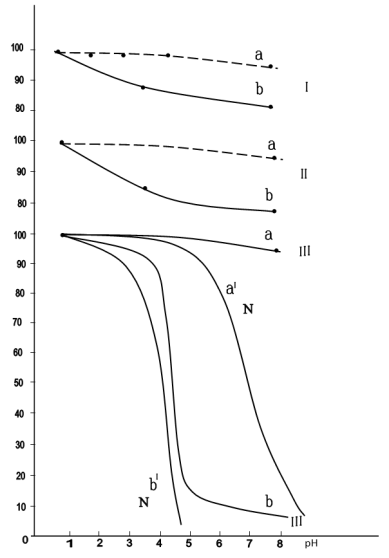
Fig. 1. Change of assimilable nd woter soluble forms Р2О5 depending on degree of NAE ammonization and norm of Hum NН4/СаО
a — assimilable form Р2О5 (Р2О5 assim/ Р2О5 общ., %);
b — water form Р2О5 (Р2О5 water./ Р2О5 общ., %);
I — СаО/ Р2О5 = 1,5 ÷ 3 phosphate raw material under Hum NН4/СаО >1
II — СаО/ Р2О5 = 2,7; Hum NН4/СаО = 0,86
III — СаО/ Р2О5 = 2,7; Hum NН4/СаО = 0,73
IV- СаО/ Р2О5 = 2,7; Hum NН4/СаО = 0,57
Table2
Influence of ratio![]() in phosphate raw material on the quantity of ammonium humate when formation of different forms of calcium phosphates
in phosphate raw material on the quantity of ammonium humate when formation of different forms of calcium phosphates
|
Ratio
|
Optimal ratioHumNH4:СаО |
|||
|
Ammonium phosphates |
Monocalcium phosphate |
Dicalcium phosphate |
Tricalcium phosphate |
|
|
1,5 |
>1 |
0,75 |
0,48 |
0,21 |
|
2,1 |
>1 |
0,82 |
0,63 |
0,44 |
|
2,7 |
>1 |
0,86 |
0,73 |
0,57 |
|
3,0 |
>1 |
0,88 |
0,75 |
0,62 |
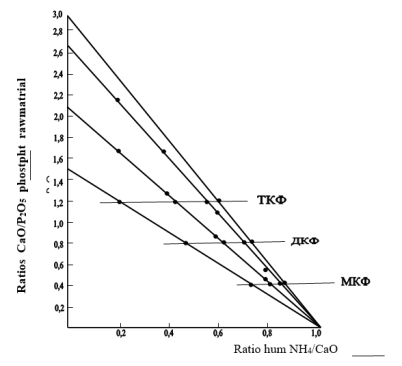
Fig. 2. Inflanse of ammonium humate amount and ratio CaO/ P2O5 in feed stoch for salt content of phosphate salts with AKB ammonisation
As shown in fig.2, with the increase of ammonium humate amount, assimilable form of P2O5 (curve 1) increases, and retrogradation degree of P2O5 reduces correspondingly.
Reduction of retrogradation degree of P2O5 assimilable form, depending on ammonium humate amount, can be explained by linking of excess part of calcium ions with ammonium humates with formation of calcium humate. This in its turn prevents from formation of unassimilable tribasic calcium phosphate. Curve 2 (fig.2) shows change of water soluble P2O5 form depending on ammonium humate amount.
With the increase of ammonium humate amount the content of water soluble P2O5 form increases, which also can be connected with formation of calcium humate.
Investigation of ammonium humate quantity influence on hygroscopic features of the product is shown (fig.2 curve3) that hygroscopic point of product increases with the increase of ammonium humate norm and reaches value of 67 % under ratio HumNH4:СаО=1:1.
This can be explained by the fact that the increase of ammonium humate amount shifts the reaction to the side of calcium humate formation and reduction of very hygroscopic calcium nitrate content, which significantly influences on hygroscopicity of finished product.
As our research shows, with the increase of ammonium humates norms in the product, the amount of organic substance increases, the content of P2O5 reduces, though the total sum of nutricious components doesn’t change. Therefore, large additives of ammonium humates are objectionable, moreover if they don’t make substantial changes to the physicochemical features of finished product.
As a result, optimum norm of amonium humates is set, namely, under wight relation HumNH4:СаО=1:1 high assimilability of P2O5 not less than 80 % and sum of nutricious components 20–30 % are remained in the product.
References:
- Beglov B. М. State and prospect of production and application of mineral fertilizers in Uzbekistan. Chemical industry today. 2003, N2, С. 25–31.
- Khudaybergenov А. К., Mirzaev F. М. Ammonation of nitric acid solutions of phosphates with the presense of cotton cuttings oxidation products. Tashkent/ 1982, — 4p –Dep in ON II TIXI, Cherkes 28.04.82
- Analysis methods of phosphate raw material, phosphoric and combined fertilizers, feed phosphates. М., «Chemistry», 1975.







