Piper nigrum is long — term industrial tree with high economic value but very sensitive to pathogens, especially Meloidogyne spp. We want to select the strains of Trichoderma sp. to control harmful nematodes on Piper nigrum. From 30 acres of pepper land in Phu Thinh town, Tam Ky city, Quang Nam province, Vienam, 6 strains of Trichoderma sp. which can resistant to nematodes were isolated. In which, the T.04 strain had the highest resistance to nematodes with 91.64 % nematode egg being suppressed.
Key word: Piper nigrum , Meloidogyne spp.; Trichoderma sp.
- Introdution
Pepper ( Piper nigrum L.) is a spice tree with high economic value, grown in many places in the world. Currently, there are about 140,000 hectares of pepper growing in Vietnam [20]. According to preliminary statistics of the General Department of Customs, Vietnam’s export volume of pepper in 2019 increased by 22 % compared to 2018, reaching 283,836 tons [19]. However, in recent years, pepper production in many regions in Vietnam is suffering from severe losses due to late-blighting yellow leaf disease, which is mainly caused by the Meloidogynespp. [1]. Meloidogyne spp. is also involved in the production of complexes with fungi besides self-pathogenicity.
Nowadays, controlling nematodes on pepper plants are mainly by chemical methods. The long-term use of the drug will cause an imbalance in the soil micro-biota, leading to outbreaks of other diseases and especially environmental impacts, as well as drug residues in agricultural products. One of the directions for preventing and controlling pathogens in pepper has been researched by scientists and has many prospects: using microorganisms, especially actinomycetes and fungi. In which, Trichoderma sp. is a potential biological control agent of plant-parasitic nematodes [6].
Therefore, the selection and assessment of the antagonistic ability of Trichoderma sp. with Meloidogyne spp. causing disease on pepper tree is an essential issue, which is the prerequisite for further research to contribute to reducing the rate of yellow leaf disease dies slowly in a biological direction in a biological way, leading to reduced economic losses and improved productivity and quality of pepper, but still ensure environmental issues.
- Materials and Methods
2.1. Collect soil samples and isolate Trichoderma sp.
Soil samples were collected at a depth of 5–20 cm (300 g/acre) around the pepper tree stump in Phu Thinh town, Tam Ky city, Quang Nam province. Soil samples were stored in plastic bags, labeled, taken to the laboratory, and stored at 4°C.
The soil sample was diluted 5 times in sterile distilled water. After that, 0.5 ml of the diluted solution was applied to 5 plates of TSM medium ( Trichoderma Specific Medium ). The plates were incubated at 28 ± 2°C for 96 hours. Different forms of morphology were transferred to Potato Dextrose Agar (PDA) medium. The identification of Trichoderma sp. was preliminary based on morphology [8].
2.2. Isolation of Meloidogyne spp.
Washing 5g of each pepper root, cut segments into of 0.5 cm. Put in a flask, add sodium hypocloite of 1 %, and shake for 15 minutes. Take out and rinse with water 3 or 4 times. Put in a mortar, crush with 250 ml of clean water. Nematode was filter by 40 µm of filter by static filtration method [12].
2.3. Culturing Meloidogyne spp.
Rooted nematodes Meloidogyne spp. bred on tomato plants as described of López-Pérez et al. [5] with minor modifications:
Tomato seeds (using susceptible varieties to Meloidogyne spp. ) were surface sterilized with 70ºC alcohol for 5 minutes, filtered out and washed with Sodium hypochlorite solution 1 % (NaOCl) for 15 minutes to exclude infectious agents outside the seeds. Then, tomato seeds were rinsed several times with distilled water to remove the NaOCl. These treated seeds will be sown in disinfected soil trays until they grow into plants with two real leaves, transfer it to a soil pot (250 cm3 soil). Finally, when tomatoes rooted, proceed nematode strains with 200 larvae/pot. After 30–60 days, nematodes can be collected.
Tomato root was infected with the nematode root Meloidogyne spp. Then, cut them into 1–2cm segments, added to NaOCl solution 1.5 %, and shake well for 5 minutes to break the gelatin shell structure of the egg sac. Larvae were collected through 40 µm sieve and eggs were collected from 25 µm sieve [13].
2.4. Assessing parasitic characteristics of Trichoderma sp. strains on nematodes
Trichoderma sp. were cultured on PDA, after 3–9 days, peak a piece of agar containing Trichoderma sp. on other medium water agar (WA). After that, thirty nematode eggs were placed on WA medium plate containing Trichoderma sp. and incubate at 25ºC. The ability of parasitic characteristic of Trichoderma sp. was observing by using the microscope for 1, 2 and 3 days. The level of fungal parasites is classified into 4 levels based on level of nematode eggs being parasitized: (i) High parasite (+++): over 80 %; (ii) average parasite (++):50–80 %; (iii) Weak parasites (+): less than 50 %; and (ix) Non-parasitic (-): 0 % [2].
2.5. Pilot — scale of nematodes eggs — antagonism
Soil pre- treated by autoclave were put into 10 x 10 cm PE plastic bags and then planting one pepper tree on each bags, taking care them until 3 months.
Trichodermasp. strains with highest egg-antagonistic ability were fermented until obtaining 10 9 CFU.ml -1 . 2 ml of cultivated medium diluted by 200ml of water was poured on each bag, and then infected 100 nematode eggs into bag containing 3 months pepper tree. Counting the number of swelling knots on roots of pepper plant after 8 weeks [2].
The experiment consisted of 4 formulas (CT). Each included 30 pepper plants:
CT1: no nematode strains
CT2 (negative control): only nematode strains
CT3: Trichoderma sp . + nematodes
CT4 (positive control): Commercial Trichoderma sp. + nematodes
2.6. Data analysis
All experiments will be performed in triplicate. The obtained results are shown as the average and standard deviation (SD). The excel software (2010) will be used to analyze the obtained data. The significant test was set at p ≤ 0.05.
- Results
3.1. Isolation of Trichoderma
From 30 acres of pepper land in Phu Thinh town, Tam Ky city, Quang Nam province, we have isolated 6 fungal strains on the TSM medium. Based on observation of some characteristics of colonies, morphology, branches, spore, mycelium, and classification table of Gary J. Samuel (2003), Kubbick và Harman (1998) and M. A. Rifai (1969), the results showed that there are 6 strains of fungal belonging to Trichoderma genus, marked from T.01 to T.06.
|
|
|
|
|
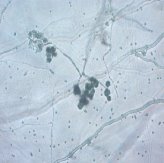
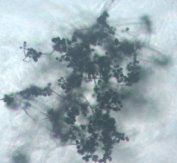
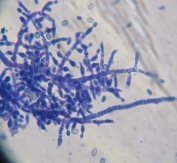
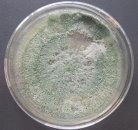
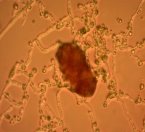
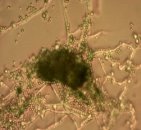
Fig. 1: Mycelium and spores of Trichoderma |
||
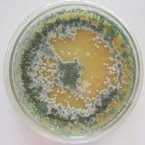
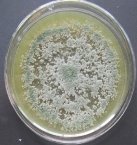
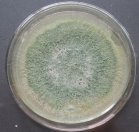
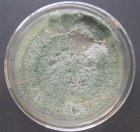
All isolates had the same characteristics of Trichoderma spp (Table 1, Figure 2): colorless mycelium, spores peduncle split into many branches, at the end of the branches develop into a round block with bare spores without baffles, colorless, linked together into small clusters at the tip of the branches by mucus. The colonies were greenish — white to green, yellowish — green, light green to dark green.
Table 1
Development of Trichoderma strains after 5 days of culture
|
Strains |
Colony |
Development of mycelium |
Pigment production |
|
T.01 |
Light green |
++ |
yellow pigments |
|
T.02 |
Dark green |
+ |
yellow pigments |
|
T.03 |
Light green |
++ |
yellow pigments |
|
T.04 |
Yellowish green |
+++ |
- |
|
T.05 |
Yellowish green |
+++ |
- |
|
T.06 |
Yellowish green |
++++ |
- |
Note:
++++: Very strong; +++: strong; ++: fair; +: Growth weak, -: negative
|
|
|
|
|
T.01 |
T.02 |
T.03 |
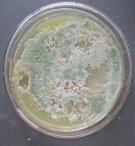
Fig. 2A: Colonies of Trichoderma sp. producing pigments (T.01, T0.2, T0.3)
|
|
|
|
|
T.04 |
T.05 |
T.06 |
Fig. 2B: Colonies of Trichoderma sp. non-producing pigments (T.04, T.05, T.06)
3.2. Assessing parasitic characteristics of Trichoderma sp. strains on nematodes
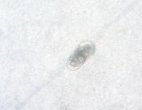
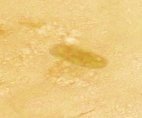
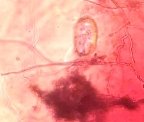
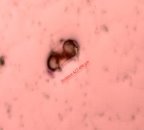
We conducted research to test the parasitic ability of 6 Trichoderma sp. strains on eggs of nematode Medoilogyne spp. The results are presented in table 2 and figure 3. From the results in Table 2 and Figure 3, all 6 Trichoderma strains isolated have the ability to parasitize nematodes eggs, in which, T.04 had the highest parasitic ability at the rate of 91.65 % and strains T.01 had the lowest parasitic with the rate of 41.65 %.
Compared with the results of Do Thi Kieu An et al (2015), the parasitize ability of Trichoderma on nematode eggs was 0.85 % lower. However, the T.04 strain has more parasitize ability than the T2 strain of Hadi Golzari et al, the rate of parasite 88.7 % was 2.95 %.
Trichoderma has been proved in many studies which is capable of living on nematode. Results of Windham et al. (1989); Sharon et al. (2001), Trichoderma have been shown to be effective against root node nematodes. In the culture media, proteolytic and chitinolytic enzymes produced by Trichoderma which could break the eggshell, although the ability to chitinolytic is probably the most important activity [9, 16].
Table 2
Parasitic ability on nematode eggs of fungal strains
|
Strain |
Number of nematode eggs being parasitized |
Percentage (%) |
Level |
||
|
Day 1 |
Day 2 |
Day 3 |
|||
|
Control |
0,00±0,00 |
0,00±0,00 |
0,00±0,00 |
0 % |
- |
|
T.01 |
4,3±2,33 |
6,0±3 |
8,33±0,33 |
41,65±2,3 % |
+ |
|
T.02 |
11,33±0,33 |
13,67±1,33 |
17,0±4 |
85±3,15 % |
+++ |
|
T.03 |
7,33±2,33 |
9,77±4,33 |
12,34±2,33 |
61,7±3,1 % |
++ |
|
T.04 |
9,33±0,33 |
16,33±6.33 |
18,33±2,33 |
91,65 ±1,7 % |
+++ |
|
T.05 |
5,66±1,33 |
7,66±0,33 |
12,6±4,33 |
63±2,6 % |
++ |
|
T.06 |
11,67±4,33 |
12,66±2,33 |
14,33±2,33 |
71,65±2,17 % |
++ |
|
Strain |
Day1 |
Day 2 |
Day 3 |
|
T.01 |
|
|
|
|
T.02 |
|
|
|
|
T.03 |
|
|
|
|
T.04 |
|
|
|
|
T.05 |
|
|
|
|
T.06 |
|
|
|
|
Control |
|
|
|
Fig. 3. Trichoderma strains parasitized on nematode eggs
From the results in Table 2 and Figure 3, all 6 Trichoderma strains isolated have the ability to parasitize nematodes eggs, in which, T.04 had the highest parasitic ability at the rate of 91.65 % and strains T.01 had the lowest parasitic with the rate of 41.65 %.
Compared with the results of Do Thi Kieu An et al (2015) [2], the parasitize ability of Trichoderma on nematode eggs was 0.85 % lower. However, the T.04 strain has more parasitize ability than the T2 strain of Hadi Golzari et al [3], the rate of parasite 88.7 % was 2.95 %.
Trichoderma has been proved in many studies which is capable of living on nematode. Results of Windham et al. (1989) [17]; Sharon et al. (2001) [15], Trichoderma have been shown to be effective against root node nematodes. In the culture media, proteolytic and chitinolytic enzymes produced by Trichoderma which could break the eggshell, although the ability to chitinolytic is probably the most important activity [9, 16].
3.3. Pilot — scale of nematodes eggs — antagonism
T.04 had the highest parasitic ability which was used for this experiment. The results were recorded in Table 4.
Table 4
Swelling knots on the 3 months pepper roots
|
Formula |
Number of swelling points |
|
CT1 |
0 |
|
CT2: Negative control |
192,3 ± 5,508 |
|
CT3 |
55 ± 3,7 |
|
CT4: Positive control |
42 ± 1,73 |
From the results in Table 4, there was a significant difference between the proportion of swelling points on the roots in non-fungal treatments (CT2) and those with fungal treatments (CT3, CT4). In CT2, the total of swelling knots was highest with 192 points. Comparing to commercial products (CT4), the figure of CT3 was higher but not different. Thus, the T.04 strain had the ability to reduce the proportion of swelling knots on pepper trees infected with nematodes.
4. Conclusion
From 30 acres of pepper land in Phu Thinh town, Tam Ky city, Quang Nam province, 6 strains of Trichoderma spp. with resistance to nematode were isolated. In particular, strain T.04 had the highest nematodes — antagonism ability.
References:
- Dao Thi Lan Hoa, Phan Quoc Sung, Tran Thi Kim Loang, Ton Nu Tuan Nam, Nguyen Xuan Hoa and Ta Thanh Nam, Study on late leaf yellowing disease on pepper in the Central Highlands and preventive measures, Proceedings Workshop on plant protection science to support the policy of crop restructuring in the Southern and Central Highlands provinces on June 26–27, 2003 in Vung Tau, 2003.
- Do Thi Kieu An, Screening and assessing the resistance of some fungal strains to the Meloidogyne incognita nematode that harm pepper plants. Proceedings of the National Plant Protection Science Conference 2015.
- Hadi Golzari, Naser Panjehkeh, Masuod Ahmadzadeh, Mohammad Salari and Ebrahim Sedaghati-khoravi, Elucidating the Parasitic Capabilities of Trichoderma against Meloidogyne javanica on Tomato, Insight Plant Disease, Volume 1 Issue 1, 2011.
- Haran S, Schickler H, Oppenheim A, and Chet I (1996). Differential expression of Trichoderma harzianum chitinases during mycoparasitism. Phytopathology 86:980–985
- J. A. López Pérez, M. Escuer, M. A. Díez Rojo, L. Robertson, A. Piedra-Buena, J. López Cepero & A. Bello Pérez, Host range of Meloidogyne arenaria (Neal, 1889) Chitwood, 1949 (Nematoda: Meloidogynidae) in Spain, Nematropica 41, 2011, 130–140
- Jatala P (1986) Biological control of plant parasitic nematodes. Annu. Pastor Phytopathol. 1986; 24: 453.
- Kubicek C. P. AND Harman G. E.(1998), Trichoderma & Gliocladium — Vol. 1: Basic biology, taxonomy and genetics. Taylor & Francis Ltd.
- Kumar K., Amaresan N., Bhagat S.,Madhuri K. and Srivastava R. C. (2012). Isolation and Characterization of Trichoderma spp. For Antagonistic Activity Against Root Rot and Foliar
- Khan A, Williams KL, Nevalainen HKM, 2004. Effects of Paecilomyces lilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biological Control 31, 346– 52.
- M. A. Rifai (1969). Revision of the genus Trichoderma . Mycological Paper 116: 1–56.
- Morton C. O., Hisch P. R., Kerry B. R. 2004. Infection of plant parasitic nematodes by nematoghagous fungi — a review of the application of molecular biology to understand infection processes and to improve biological control. Nematology, 6: 161–170 https://doi.org/10.1163/1568541041218004.
- Nguyen Ngoc Chau, 2003. Nematode nematode and control facility. NXBKHKT. Hanoi, 302 p.
- R. S. Hussey & K. R. Barker, Comparison of methods for collecting inocula of Meloidogyne spp. including a new technique, Plant Disease Reporter, 1973, 57, 1025–1028
- Sayed M. A., Tahany M. A. Abdel-rahman, A. A. Ragab and Aya A. M. Abdellatif, Biocontrol of Root-Knot Nematode Meloidogyne incognita by Chitinolytic Trichoderma spp. Egypt. J. Agronematol., Vol. 18, No.1, PP. 30–47 (2019)
- Sharon E., Bar Eyal M., Chet I., Herrera E., Strella A., Klelfeld O., Splege Y. 2001. Biological control of the rootknot nematode Meloidogyne javanica by Trichoderma harzianum . Phytopathology, 91: 687–693 https://doi.org/10.1094/PHYTO.2001.91.7.687
- Tikhonov V. E., Lopez-Llorca L. V., Salinas J., Jansson H. B. 2002. Purification and characterization of chitinases from the nematophagous fungi Verticillium chlamydosporium and V. suchlasporium . Fungal Genetics and Biology, 35: 67–78 https://doi.org/10.1006/fgbi.2001.1312
- Windham. G. L., Windham, M.T., Williams. W. P (1989), “Effects of Trichoderma spp. On maize growth and Meloidogyne arenaria reproduction”, Plant Disease 73: 493–495.
- (http://vinanet.vn/thuong-mai-cha/thi-truong-xuat-khau-hat-tieu-nam-2019–724391.html
- http://thanhtra.com.vn/kinh-te/lao-dong-viec-lam/dien-tich-ho-tieu-dang-gia-dan_t114c7n152940).