Mineral anions are one of the most important toxic substances that are harmful to humans and animals even in low concentrations. Contamination of groundwater and rural drinking water by nitrates from livestock, human waste, other organic wastes or chemical fertilizers is a potential hazard worldwide. Nitrate in the body is dangerously converted to nitrite and nitroso-N compounds, which are carcinogenic and the main cause of methemoglobinemia. Nitrate in water sources is not easily separated due to its high solubility. Neonatal disease and death from nitrate-induced globinemia are often misdiagnosed, possibly as sudden infant death syndrome. Undoubtedly contributes to the national infant mortality rate.
Keywords: groundwater, contamination, nitrate, methemoglobinemia, infant death.
Минеральные анионы — одно из важнейших токсичных веществ, которые вредны для человека и животных даже в низких концентрациях. Загрязнение грунтовых вод и питьевой воды в сельской местности нитратами от домашнего скота, человеческими отходами, другими органическими отходами или химическими удобрениями представляет собой потенциальную опасность во всем мире. Нитраты в организме опасно превращаются в нитриты и нитрозо-N соединения, которые являются канцерогенными и являются основной причиной метгемоглобинемии. Нитраты в водных источниках трудно отделить из-за их высокой растворимости. Заболевания новорожденных и смерть от нитрат-индуцированной глобинемии часто ошибочно диагностируются, возможно, как синдром внезапной детской смерти. Несомненно, способствует росту детской смертности в стране.
Ключевые слова: подземные воды, загрязнение, нитраты, метгемоглобинемия, младенческая смерть.
One of the main needs of life, growth and development in any country is the existence of water. Groundwater resources, as reliable sources, provide an important part of the water needs of communities. Groundwater is water that accumulates below the surface of the earth, among soil particles and rocks in slides called groundwater voyages. These sources make up 97 % of the world's fresh water and are an important source of drinking water in many parts of the world; So that in some areas, especially areas where surface water resources are limited or polluted, are the main and only source of drinking water. Since in the past, groundwater was considered as a hidden source of water, its pollution often received less attention, but today, the potential for vulnerability of groundwater resources has received much attention and very strict rules and regulations have been set for it. But today, the rapid growth and expansion of cities and the development of industrial, agricultural, academic and tourist aspects have created new conditions for groundwater resources associated with them.In recent years in many urban areas due to the increasing use of soluble chemicals as well as the leakage of domestic and industrial wastewater into groundwater aquifers, groundwater quality has declined sharply. Bon Nitrate is one of the most important pollutants in groundwater resources and due to its high solubility, removing this contaminant from drinking water by conventional methods is very expensive. In addition to the natural level of nitrogen, nitrate enters the water and soil resources due to the entry of human wastewater, municipal and industrial wastes, as well as agricultural activities, and has adverse effects on the health of consumers.
Natural attention of Nitrate in Soil and Ground water
Nitrate pollution of groundwater is governed by natural biogeochemical processes that control nitrate leaching into groundwater. Within the root zone of the topsoil, nitrogen fixation and nitrification processes make nitrogen available to plants in the form of ammonium (NH4+) and nitrate. Nitrate reduction may occur by plant uptake, mineralization-immobilization processes, volatilization, losses by run-off and denitrification. These processes, either as stand-alone or in combination, limits nitrate flux into groundwater. Notwithstanding, nitrate ions, unlike the positively charged NH4+ (that are bound to negatively charged soil particles), are loosely bound and may percolate beyond root zones into the phreatic zones of groundwater aquifer media. Through the soil profiles, the complex biogeochemical processes have been reported to reduce the levels of nitrate reaching the groundwater. Figure 1 shows the processes of nitrogen transformation in the subsurface.
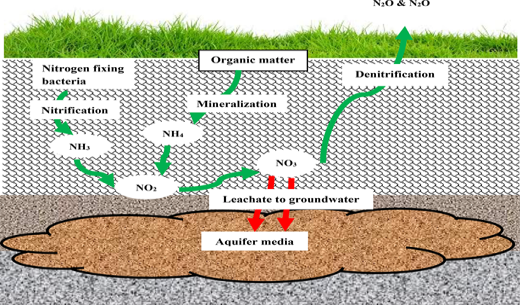
Fig. 1. Nitrate cycling in the subsurface and pathway to groundwater
Nitrate and its effect on humans
Mineral anions are one of the most important toxic substances that are harmful to humans and animals even in low concentrations. Among these anions, nitrate ion is one of the most important pollutants in surface and groundwater due to its high solubility in water. Nitrate ion has always been present in nature as part of the natural nitrogen cycle. Nitrogen compounds are converted to nitrate by bacteria in oxidized soil, and the nitrate produced can penetrate into aquifers. The concentration of nitrate naturally in groundwater sources is a few milligrams per liter, so that according to reports in the United States, the concentration of nitrate in the normal state is between 4 and 9 milligrams per liter. In general, the sources that increase nitrate in groundwater are very high, and sometimes the presence of these factors can increase the cause of nitrate in very high amounts (for example 1500 mg / l in India), when Excessive nitrate concentration in drinking water Drinking water is the most important entry of nitrate into the body.
The average nitrate uptake in humans varies from 43 to 131 mg / L. The total amount of nitrate absorbed in the human body is estimated at 30 to 268 mg / L per day, depending on the amount excreted in the urine. Nitrate absorbed by the body causes adverse effects for humans, especially in infants and pregnant women. Nitrate in drinking water in the body is reduced to nitrite and eventually converted to nitrogenous compounds, which are highly toxic and carcinogenic compounds. In this regard, the need to define the standard seems very necessary, so that most countries or organizations and departments of health and the environment have defined comprehensive and diverse standards in the field of various pollutants. Also, in the case of nitrate and nitrite pollutants, attention and health-related concerns caused by this issue, has led to their maximum amount along with the environmental standards of the world. According to the standards in Afghanistan and the world, the acceptable amount of nitrate in drinking water is listed in Table (1–1).
Table 1
National and International Nitrate density in drink water.
|
Name of standards |
Maximum allowable ion nitrate mg/l |
|
Standard for Afghanistan WHO US Environmental Protection Agency(EPA) Europe Environmental Agency(EEA) |
50 45 45 50 |
Conclusion: Since groundwater was considered as a hidden source of water in the past and was relatively far from reach, but with the change of climate and reduction of surface water, the process of its use began many years ago, but this source due to population increase, Aspects of agriculture, industry, and production wastes have exceeded the high capacity of this resource and polluted groundwater resources, which poses a serious threat to the health of consumers, including dangerous bacteria that In water, nitrates are caused by contaminants in the water, which have been very harmful so far, causing cancer and hemoglobin in pregnant women and children.The global impact of nitrate pollution in most cases of groundwater aquifers is the issue that is the most important health and environmental concerns, so should find the way to solve the main problem to live comfortable.
References:
- Shariatmadari.N, Mohsen.S, Hassam.D.2010. “Investigation of hexavalent chromium removal (VI (Cr) Contaminated clay using simultaneous combination of electrokinetic and iron nanoparticles as reactive wall, Permeable, (PRB (Quarterly Journal of Environmental Science and Technology” 09 (3) 70–56.
- Acar, Y. B., J. T. Hamed, A. N. Alshawabkeh, and R. J. Gale. 1994. “Removal of Cadmium (II) from Saturated Kaolinite by the Application of Electrical Current.” Géotechnique 44 (2): 239–54.
- Pro. N. Eqrar. (2021) Resources, “Hydropolitics and Water Structure of Afghanistan”,(77–145).
- Solomon K. M. Huno;Eldon R. Rene;Eric D. van Hullebusch;Ajit P. Annachhatre., Journal of Water Supply: Research and Technology-Aqua (2018) 67 (8): 885–902. https://doi.org/10.2166/aqua.2018.194 .
- Gharminia. Mahdyar.2017.” Permeable Reactive Barrier (PRB), Performance in Removing Nitrate from Groundwater”. (5–32).
- Шурэнцэцэг Хурэлбаатар.2009.”Кочество питьевой воды при разлыных способых водоподготовки”,”диссертации на соискание ученой степени кандидата химических наук”.Сраница(20–35).
- https://www.stonybrook.edu/commcms/cleanwater/research/PRB %20white %20paper_FINAL.pdf.
- Nitrate and Nitrite in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality World Health Organization 201. (9–11).







