Recently, more attention has been paid to the leaching of heavy metals from the sediment. The leachability and bioavailability of trace metals in sediments depend on their chemical and physical associations (Singh et at., 2000), thus their mobility depend on several factors: the sediment type on the basis of the parameters that affect metal interaction, basically pH, CaCO 3 , CEC, nutrient status, organic matter content, redox potential and texture; the nature of the contamination in terms of origin and characteristics of the deposition an composition; and the environmental conditions that may lead to weathering, such as acidification, redox processes, temperature and water regime. Metals in sediment may form specific mineral phases, be loosely bound on exchangeable sites, co-precipitate, be adsorbed onto mineral phases, be fixed by organic matter and sulfides or be structurally bound in alumino-silicate structures (Belzile et at., 1989).
The objective of this study was to evaluate the heavy metal remove capacity of sediment by using different solutions and to compare the heavy metal remove capacity between different solutions.
Materials and methods
1. Materials
Total 12 sediment samples were collected at To-Lich, Kim-Nguu river systems, Hanoi, Vietnam (which are the main source of irrigation water for suburban agricultural land and the source of aquaculture pond). Sediment samples were collected from the surface zone (20 cm) of the sediment (SD7, SD9, SD 11,SD16, SD18, SD19) at the 6 sites submerged with water. The core samples of 90 cm depth were also taken at 3 sites (SD1, SD2, SD3, SD13, SD14, SD15) where sediment surface is exposed to the air.
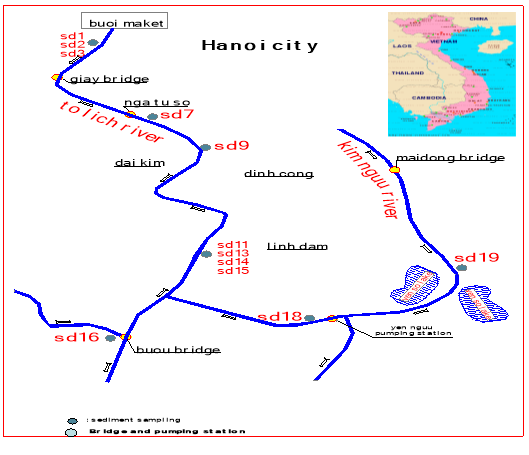
Fig. 1. Site sampling location
2. M ethods
Total heavy metals in the soil was digested with 1 M HNO 3 at 96 0 C for an hour and centrifuged. The 0.1 M HCl and 0.1 M HNO 3 solutions were used to extract available heavy metals with ratio is 1/5 the suspension was agitated continuously for 1 h and centrifuged at 2.500 rpm for 10 min. The dissolved metals in the supernatant were analyzed by atomic absorption spectrophotometer (Committee of Soil standard methods for Analyses and Measurement, 1986). Leaching test followed the same procedure as the batch adsorption test, the soil to solution ratio of 1:10. Distilled water (pH 5.5) and nitric acid (HNO 3 ) and acetic acid (CH 3 COOH) at pH 4 were used as the leaching solutions, respectively, to simulate normal precipitation and acid rain conditions, also Ethylene di amino tetra acid (EDTA) 1M was used (Li, 2002).
Results and discussions
1. Total heavy metal of sediment
The total heavy metal of the sediment samples was shown in table 1. The total heavy metal concentration varied considerably among the samples: 220 to 475 mg/kg for Cu, 260 to 665 mg/kg for Pb, 250 to 535 mg/kg for Zn, 2.5 to 40 mg/kg for Cd, 505 to 655 mg/kg for Cr, and 48 to 165 mg/kg for Ni.
Table 1
Total heavy metal concentration of sediment samples
|
No |
Total heavy metal concentration (mg/kg) |
|||||
|
Cu |
Pb |
Zn |
Cd |
Cr |
Ni |
|
|
SD 1 |
260 |
368 |
250 |
4.4 |
540 |
65 |
|
SD 2 |
320 |
395 |
441 |
5.3 |
550 |
63 |
|
SD 3 |
345 |
425 |
345 |
5.2 |
525 |
48 |
|
SD 7 |
472 |
490 |
478 |
20.0 |
505 |
165 |
|
SD 9 |
445 |
450 |
490 |
40.0 |
517 |
108 |
|
SD 11 |
357 |
348 |
422 |
2.7 |
638 |
57 |
|
SD 13 |
340 |
280 |
395 |
2.5 |
637 |
52 |
|
SD 14 |
220 |
260 |
370 |
3.7 |
615 |
58 |
|
SD 15 |
240 |
370 |
325 |
2.7 |
605 |
50 |
|
SD 16 |
401 |
435 |
324 |
8.5 |
560 |
64 |
|
SD 18 |
415 |
430 |
535 |
17.0 |
655 |
142 |
|
SD 19 |
475 |
665 |
520 |
3.5 |
585 |
151 |
2. Leaching of sediment
Potential leachability of using different solutions H 2 O, HNO 3 , CH 3 COOH and EDTA (expressed as the percentage of total metal content) were calculated by total of 5 times leaching test concentration.
The heavy metals leaching concentration of sediment was showed on figure 2. In general, potential leachability decreased in the order: Cd > Ni> Zn> Cr> Cu> Pb. We observed a high potential release of Cd. Carbonates and Oxides may be considered a major solubility controlling solid phase for these metal. In fractionation, we observed that a large proportion of Cd in the sediment was present in the Oxidation. In the upland disposal environment, trace metal solubility as a function of pH is directly released to pH-controlled dissolution and desertion process, which may also involve initially present sediment phase and phase formed during subsequent drainage and oxidation of sediment.
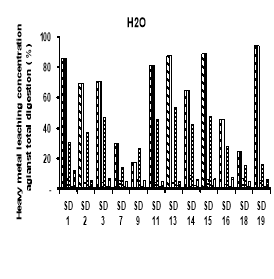
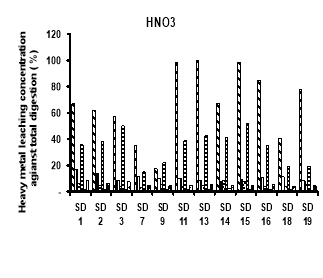
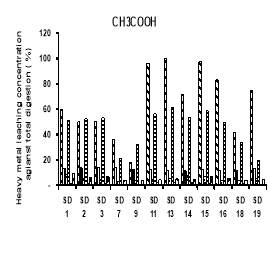

Fig. 2. Heavy metal leaching concentration of sediments by different chemical using
The heavy metals leaching by using H 2 O decreased in following order: Cd (17.4- 94 %) > Ni (14.18- 53.85 %) > Cr (7.33–16.94 %) > Zn (4.65–12.36 %) > Cu (3.09–8.86 %) > Pb (0.88–2.47 %). The Cd concentration is also the highest concentration of leaching metals when using HNO 3 , ranged of 17.70 to 100 %, followed by Ni (15.03–51.80 %), Cr (7.33–16.94 %), Zn (4.02–8.52 %), Cu (3.01–8.55 %) and Pb (0.57–2.20 %). The order of heavy metals leaching concentration when using CH 3 COOH was Cd (18.38- 100 %), followed by Ni (19.14–61.54 %), Cr (11.13–14.44 %), Zn (3.73–9.44 %), Cu (3.05–8.58 %) and Pb (0.36–2.21 %). There is slightly different in order when using EDTA, Cd (19–100 %) is the highest leaching concentration, then Ni (17.39–87.92 %), third one is Zn (12.94–59.04 %), followed by Cu (11.87–27.27 %), Cr (11.65–24.37 %) and Pb (4.49–59.04 %).
3. Comparison the heavy metal removes capacity between different solutions
The demonstrate the potential of heavy metal release in the winter environment, the rainy season and acid rain situation, the leaching test was used to determine the release capacity of heavy metal. This permits one to determine how strongly the heavy metal is held by the sedimens. Thus H 2 O, HNO 3 , CH 3 COOH and EDTA were introduced. The results of leaching test on the originally heavy metal contaminated sediment are shown in figure 4.
Average heavy metal leaching concentration of sediment soils by using EDTA was 70.05 % of Cd; 46.70 % of Cr; 17.96 % of Cu; 59.48 % of Ni; 7.21 % of Pb; and 32.96 % of Zn, and it was quite higher than that of using others chemical. It can be improved that, in generally, EDTA had relatively highly leachability released of all heavy metals compared to CH 3 COOH, HNO 3 and H 2 O for sediment. EDTA and acetic acid provide additional information on the effect of complexation and acidification processes on metals extractability.
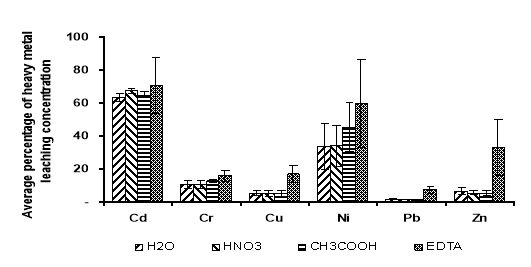
Fig. 3. Average percentage of heavy metal leaching concentration for each solution of the sediment at all sampling sites. The variation of heavy metal concentration among the sampling sites is also indicated
Sahuquillo at al (2003) found that higher percentage are extracted with EDTA in comparison others chemical, and his explained that EDTA extracted both carbonate- fraction and organic fraction of metals in sediment with low organic matter content. The heavy metals show higher leaching value with EDTA, which indicated that these heavy metals are more easily remobilized by complexion than acidification process. Thus, as use of EDTA would assure better analytical performance during the measurement because of its higher extraction capacity.
Conclusion
Potential leachability of using H 2 O, HNO 3 , CH 3 COOH and EDTA leaching chemicals decreased in the order: Cd > Ni> Zn> Cr> Cu> Pb for sediment; EDTA had relatively highly leachability released of heavy metal compared to CH 3 COOH, HNO 3 and H 2 O for sediment.
References:
- Belzile, N., N. Lecomte, and A. Tessier (1989), Heavy metal extractability in long term sewage sludge amended soils, Environment Science and Technology 23.
- Committee of Soil Standard Methods for Analyses and measurements, (1986) Standard Methods for Analyses and measurements. Hakuyusha. Tokyo
- Li YL, (2002), Environmental fate of lead in surface soils along highway corridors at Willingdon and Trans Canada Highway #1, Ministry of Transportation and highways Engineering Branch 4B-940 Blanchard Street Victoria, B. C. V8W 9T5
- Sahuquillo A, Rigol A, Rauret G, (2003), Overview of the use of leaching /extraction tests for risk assessment of trace metals in contaminated soils and sediment, Trends in Analytical Chemistry: 22(3), 152–159
- Singh SP, Ma LQ, Tack FMG, Verloo MG, (2000), Trace metal leachability of land –disposed dredged sediments,Journal of Environmental Quality: 29 (4), 1124–1132.







