We were observed transformation 4-ethylpyridine and 2-methyl-5-ethylpyridine by fungus Beauveria bassiana ATCC 7159. Stereoselective oxidation of methylene group leading to the optically active (-)-(1-hydroxyethyl) pyridine was shown. Besides, the hydroxylation of methyl groups and the oxidation of the heterocyclic ring in the nitrogen atom to the respective primary alcohols and N-oxides were observed.
Keywords: transformation, 4-ethylpyridine, 2-methyl-5-ethylpyridine.
Introduction
Some fungi are able to hydroxylate pyridine alkyl substituents without affecting the heterocyclic ring. In this way, the corresponding hydroxyalkylpyridines of pharmacological interest can be obtained. Not many strains of microorganisms are known to be capable of hydroxylation of pyridines.
Previously, we studied the transformation of 2-ethylpyridine by the fungus Beauveria bassiana ATCC 7159 [1]. In the result of researches was obtained the hydroxylated derivative of the initial substrate. The yield of the product was observed as 60 %. The aim of this work was to study the possibility of hydroxylation of 4-ethylpyridine and 2-methyl-5-ethylpyridine by the fungus B. bassiana ATCC 7159.
Materials and Methods
We were used the strain of fungus Beauveria bassiana ATCC 7159 from the American Type Culture Collection.
The process of hydroxylation was carried out in a buffer solution of pH 6.0, for 48 hours by suspension of non-reproducing cells which previously grown up to stationary phase in the medium of the following composition (g / L): glucose — 20.0; corn steep liquor — 10.0; peptone — 5.0; KH 2 PO 4– 5.0; and deionized water, 1000 ml; the pH was adjusted to 5.0. Substrates for transformation were added to the buffer mixture in an amount of 100 mg / L. The products of transformation were extracted from the culture medium by extraction with chloroform and separated on a column in the solvent system — hexaneethyl acetate-methanol (5:5:1). For column chromatography was used silica gel — Kieselgel 0.200±0.036 (Merck AG, Germany). Thin-layer chromatography was performed on plates Silica gel 60 F 254 (Merck KgaA, Darmstadt, Germany).
Electron ionization (EI) mass spectrometry was performed at an electron energy of 80 eV on the instrument Varian MAT-112 and on the instrument MX 1321A with the electron energy of 70 eV.
Optical rotations were measured on a polarimeter Chemapol IV (Rudolph, USA).
Results and discussion
It was found that the transformation of 4-ethylpyridine (I) proceeded with the formation of (-)-4-(1-hydroxyethyl)pyridine (II) in a yield of 3.5 %, 4-(2-hydroxyethyl)pyridine (III) in a yield of 3.8 %, and N-oxide (IV) in a yield of 0.6 % (Fig. 1).
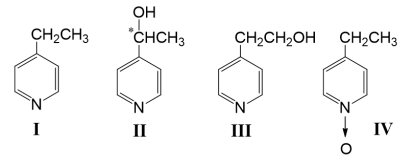
Fig. 1. Structures of 4‑ethylpyridine (I) and products of transformation
(-)-4-(1-hydroxyethyl) pyridine (II); [α] D 20 –36,3 ° ( c 4.27, CH 3 OH); melting point 63–65°C (petroleum ether), literature data [2]; Rf =0.24. UV spectrum: ƛmax 257, 277 nm literature data [2]. The mass spectrum of compound II (m/z, I %):123 (31), 122 (10), 108 (57), 106 (23), 81 (35), 80 (100), 78 (44), 52 (38), 51(64).
4-(2-hydroxyethyl) pyridine (III) — colorless oil; Rf =0.35; UV spectrum: ƛmax 257 nm. The mass spectrum of compound III (m/z, I %): 123 (50), 122 (10), 106 (12), 105 (10), 93 (100), 92 (21), 78 (27), 66 (23), 65 (27).
N-oxide (IV) — colorless oil; Rf =0.09; UV spectrum: ƛmax 265 nm. The mass spectrum of compound IV (m/z, I %): 123 (59), 108 (100), 107 (41), 106 (38), 92 (21), 91 (35), 80 (35), 79 (29), 65 (24).
During the transformation of 2-methyl-5-ethylpyridine (V) by the fungus B. bassiana ATCC 7159 were isolated three products: 3-(1- hydroxyethyl)-6-methylpyridine (VI) in a yield of 10.1 %, 2- hydroxymethyl)-5-ethylpyridine (VII) in a yield of 3.8 %, and N-oxide (VIII) in a yield of 1.4 % (Fig. 2).
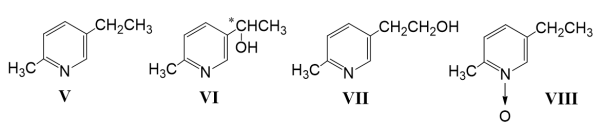
Fig. 2. Structures of 2-methyl-5-ethylpyridine (V) and products of transformation
(-)-3-(1- hydroxyethyl)-6-methylpyridine (VI); [α] D 20 –30,0 ° ( c 3.17, CH 3 OH); melting point 50–51°C (petroleum ether); Rf =0.30; UV spectrum: ƛmax 265, 272 nm. The mass spectrum of compound VI (m/z, I %):137 (26), 136 (4), 122 (100), 120 (8), 94 (50), 93 (18), 92 (7), 78 (7), 65(7).
2- hydroxymethyl)-5-ethylpyridine (VII); melting point 88–89°C (petroleum ether); Rf =0.40, UV spectrum: ƛmax 267 nm. The mass spectrum of compound VII (m/z, I %):137 (61), 136 (100), 122 (39), 108 (94), 107 (30), 106 (33), 93 (87), 78 (27), 77 (33).
N-oxide (VIII) — colorless oil; Rf =0.51; UV spectrum: ƛmax 260 nm. The mass spectrum of compound VIII (m/z, I %): 137 (100), 121 (33), 120 (73), 106 (43), 105 (4), 93 (69), 91 (33), 78 (15), 77 (51).
Not many works are known from the literature about the microbial preparation of isomeric (-)-(1-hydroxyethyl) pyridines [3–6]. The processes described here have clear advantages over the known chemical methods for the preparation of such compounds as by reducing the number of stages and also by eliminating expensive and aggressive reagents from the process.
It is known that some derivatives of optically active (1-hydroxyethyl)pyridines have antihistamine activity, and the (-) enantiomers, which can be used as antiallergic drugs, are more active [7].
References:
- Parshikov I. A., Khasaeva F. M. Bioconversion of 2‑Ethylpyridine by Beauveria bassiana. // Young Scientist. 2015. N 15(95). P.241–243.
- Gottarelly G., Samori B. Optical activity of the pyridine chromophore // Tetrahedron Lett. 1970. N 24. P.2055–2058.
- Parshikov I. A. Microbial conversions of nitrogenous heterocycles. 2015. M.: Editus, 130p.
- Parshikov I. A., Terentyev P. B., Modyanova L. V. Microbiological transformation in a series of nitrogen containing-heterocycles. (Review) Chemistry of Heterocyclic Compounds, 1994, 30, N11–12, 1308–1330.
- Modyanova L. V., Duduchava M. R., Piskunkova N. F., Grishina G. V., Terentyev P. B., Parshikov I. A. Microbial transformations of piperideine and pyridine derivatives. // Chemistry of Heterocyclic Compounds, 1999, 35, N5, P. 580–586.
- Holland H. L., Morris T. A., Nava P. J., Zabic M. A new paradigm for biohydroxylation by Beauveria bassiana ATCC 7159. // Tetrahedron. 1999. V.55. N 24. P.7441–7460.
- Tilford Ch.H., Shelton R. S., Van Campen M. G. Histamine antagonists. Basically substitued pyridine derivates // J. Am. Chem. Soc. 1948. V.70. P.4001–4006.







