This paper examines the use of the main methods for the industrial production of mellitic acid, using different starting substances and production processes, which have disadvantages that require careful analysis to find a rational production method for today.
Keywords: mellitic acid, methods, rational, industry, disadvantages.
В данной работе рассматривается использование основных методов промышленного получения меллитовой кислоты, использующие различные исходные вещества и процессы производства, которые имеют недостатки, требующие тщательного анализа для поиска рационального метода производства в наши дни.
Ключевые слова: меллитовая кислота, методы, рациональный, промышленность, недостатки.
Mellitic acid is a six-base acid of the aromatic series with the formula C 6 (COOH) 6 (Fig. 1).
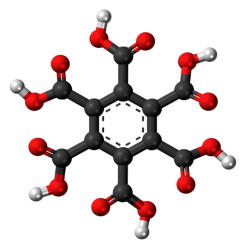
Fig. 1. Structural formula of mellitic acid
Up to 80s of 20th century there was no industrial method of production of mellitic acid, but possibility of wide use of its derivatives attracted attention of chemists, therefore there are enough researches on development of method of its production [1], [3], [4]. Mainly methods based on oxidation of pretreated coal or graphite, including electrochemical process are considered [3] (Fig.2).

Fig.2. Oxidation of pre-treated coal or graphite to produce mellitic acid
Currently, the most common way to produce mellitic acid is two-stage oxidation of pre-crushed fossil coals or products of their heat treatment. For this purpose, fossil coal or coal after heat treatment at 600–650oC in presence of 1,5–2 % AlCl3 is firstly oxidised with concentrated nitric acid in proportion 1:15 at temperature 108–110oC during 18 h. Then the nitric acid is stripped off and a 25 % aqueous NaOH solution is added to the dry residue. In the second oxidation step a current of chlorine gas is added to the reaction mixture until the solution reacts neutrally and turns yellow-green. The mellitic acid is extracted on the sulfopolystyrene cation exchange, the eluate is evaporated in a water bath, the dry residue is recrystallized from nitric acid. The yield of mellitic acid is 4,5–33,9 % with 0,3 % of nitrogen impurity and 0,2 % of mineral impurity [2].
The main disadvantages of this method are:
— multistage oxidation process taking place in acidic, alkaline and neutral media;
— high corrosiveness of the nitric acid oxidiser and the need to handle toxic gases — chlorine and nitrogen oxides
— need for hazardous handling of hot nitric acid; — extremely low mellitic acid yield (less than 1 g per 5 litres of nitric acid);
— the necessity to prepare nitric acid with a concentration of more than 80 % due to a lack of acid with this concentration;
— necessity of additional heat treatment of coal at 600–650oC to obtain mellitic acid yield of more than 4.5 %;
— The presence of impurities in the target product.
The method consisting in electrochemical anodic oxidation of carbon-graphite materials to mellitic acid is the closest to the most rational method with respect to technical essence and the result achieved. The process is carried out by direct oxidative destruction of carbon-graphite anode in 0.05–1 M H 2 SO 4 solution, temperature 75–95 °C, potential 1.05–1.3 V. The rate of formation of the target product was on average 0.17 g/h per litre of solution. After oxidation process completion solution is filtered from sludge, evaporated to 1/10-th volume, centrifuged, precipitated graphite oxide is filtered, treated with ammonia solution to get insoluble ammonium salt of melliteitic acid from which during acidification with concentrated nitric acid pure melliteitic acid is produced as colourless needle-like crystals with 20–30 % yield of substance [1].
The main disadvantages of this method are:
— Need to use pre-formed carbon-graphite anode in the process, as the target product is obtained by its direct destruction in the electrolyte;
— impossibility of using fossil coal as a raw material for electrochemical oxidation, and similar materials with low electroconductivity, as anodes, formed from them, practically are not subject to electrochemical oxidation;
— The duration of the electrochemical process as only the exposed surface of the carbon-graphite anode in the electrolyte is oxidised;
— The multistage and difficulty of extracting the target product;
— Use of corrosive hot sulphuric acid solution as an electrolyte.
References:
- Galikeev A. R., Galiamov E. Z. Method of benzene-hexacarboxylic acid (mellitic acid)/Patent RF2098402, patent holder: Ufa State Petroleum Technical University, publication of patent: 10.12.1997
- Golubeva T. B., Tselischeva S. V. Catalytic systems in chemistry course. / Manuals for laboratory and practical classes for students of full-time and correspondence forms of education. — Ekaterinburg, Ural State University, 2011. — 12 с.
- Shapkin I. P., Polyakov V. Y., Kondrikov N. B. Method of benzene-polycarboxylic acids preparation / Patent RF2194034, patent holder: Far Eastern State University, publication patents: 10.12.2002
- «An inexpensive and efficient synthetic method for the preparation of pyromellitic dianhydride promoted by ionic liquid» Yu Lin Hu, Ming Lu, Xiao Bin Liu, Sheng Bin Zhang, Zhan Hui Ji, and Ting Ting Lu, Chemical Engineering College, Nanjing University of Science and Technology, Nanjing 210094, PR China.







