It was synthesized polyoxometalate containing rare earth element (Gd,Nd). The structure of the REE-POM was investigated using XRD, IR and TGA physic-chemical methods. Obtaining catalysts were applied at hydrodesulphurization of organosulfur compounds and hydrogenation of aromatic hydrocarbons. The activity of the catalyst depend on the composition and the nature of the complexes. The highest results of the proceses were obtained in the presence of neodium polyoxomolybdate.
Keywords: polyoxometalates, diesel, hydrodesulphurization.
The domestic oil refining industry is currently facing a number of serious problems, among which the most important are the following: deterioration in the quality of oils supplied for processing; insufficiently high quality of the main types of oil products; lag in the level of efficiency of catalysts of the leading catalytic processes. These problems are closely interrelated: the environmental characteristics of diesel fuels must be dramatically improved with a significant deterioration in the quality of raw materials, which is possible only with the use of highly efficient catalysts.
Since the end of the last century, there has been a significant increase in the fleet of cars with diesel engines. Only in the last 5 years, the number of cars with diesel engines has increased by 20 % [1]. The increase in demand for diesel fuel (DF) is accompanied by a tightening of requirements for its quality, which is reflected in the specifications of various countries [1–6]. Particular attention is paid to the environmental safety of diesel fuel. One of the main factors negatively affecting the environmental properties of diesel fuel is the sulfur content.
The purpose of this work is a comprehensive study of the chemical composition of diesel fractions supplied for hydrotreatment, as well as the study of catalytic transformations of sulfur-containing and aromatic compounds of diesel fractions, the selection and creation of catalysts for these processes.
Experimental part
All inorganic and organic compounds from Aldrich Chemical Co. (St Louis, MO) were used as received. The structures of the synthesised polyoxocomplexes were studied using various analysis methods.
REE (Nd, Pr)-containing polyoxomolybdates, polyotungstates were prepared by mixing aqueous solutions of 0.620 g (0.5 mmol), (NH 4 ) 6 Mo 7 O 24 4H 2 O, 0.91 g (2 mmol) Gd (NO 3 ) 3 6H 2 O, 1 g of 85 % H 3 PO 4 (8.6 mmol) and 1.5 g of mesoporous carbon material at a temperature of 85–90ºC for 8 hours. followed by evaporation of the suspension and heat treatment at 150–180ºС.The yield of catalyst is 3.2 g.
The synthesized polyoxomolybdates, polyoxotungstates were analysed using XRD, with diffractograms recorded using MiniFlex (Rigaku) X-ray diffractometer. Crystal structure of the prepared catalysts was determined using a S–3400 N scanning electron microscope equipped with an Oxford Instruments NanoAnalysis microanalysis system.
NTE-modified polyoxomolybdate and polyoxovolfromat catalysts, their samples impregnated on high-dispersion carbon material, IR spectra Fourier spectrometer Vertex (firm «Brooker», Germany) were recorded in the range of 100–4000 cm -1 .
To study the thermal properties of catalysts, their thermograms were recorded on a device Perkin Elmer, STA 6000.
For in-depth study of NTE-modified polyoxomolybdate and polyoxovolframate catalysts, IR-Fourier Lumos microscopy recorded microphotography of their surface in the wavelength range of 600–4000 cm -1 . As the device is equipped with an 8-magnifying lens, it provides an IR spectrum of selected and target points on the surface, along with a microscope image of the surface of these catalysts. In order to make the analysis results comparable and informative, their spectra in both 2D and 3D formats are presented.
Density, refractive index, fractional composition, sulfur content, content of aromatic hydrocarbons of various classes, and the amount of sulfonated compounds were determined for all diesel fractions, which are components of the feedstock for diesel fuel hydrotreatment units, and hydrogenation products.
The density of diesel fractions and hydrogenates was determined by the pycnometric method at 20°C [7]. The refractive index was determined at 20 °С according to [8]. The total content of aromatic hydrocarbons in diesel fractions and hydrogenates was determined by PC — spectroscopy in the absorption band 1550–1680 cm -1 . IR spectra were recorded on a FT-IR Bruker Alpha. A calibration curve was built for diesel fractions. To construct a calibration curve, the method of concentration of aromatic substances was used, which ensures an increase in the content of the latter up to 70–80 % wt.
Results and discussion
The structure of the synthesized catalysts, IR spectra were taken at selected points on the surface of the catalyst under a microscope with the LUMOS IR-Fourier device. For this purpose, Gd-modified polyoxomolobdate and their peroxoforms were analyzed. As an example, consider a modified peroxomolybdate catalyst with Gd. This catalyst was first microphotographed under a microscope, then 5 points were selected on the surface of the catalyst in the obtained microphotography and IR spectra were drawn for each point.
Absorption bands 829–868 cm-1, 922 cm -1 corresponding to O — Mo — O — Gd, O — Mo — O and correlation are observed in the IR spectrum of point 1. There are also absorption bands in the spectrum corresponding to the deformation (1100 cm -1 ) and valence oscillations (3303, 3597 cm -1 ) of the O — H bond (Figure 1, a). 1042, 1089 cm-1 are absorption bands corresponding to the P — O — bond. At the same time, 986 cm -1 and 690 cm -1 absorption bands are present in the spectrum, respectively, according to -O — O– and Mo — O — O bonds. Comparison of the IR spectra of points 1 and 2, 3, 4, 5 shows that they are almost identical. This indicates that the catalyst is homogeneous. A microscopic view of the catalyst surface and a comparative description of the points are given in Figure 1a and Figure 1b.
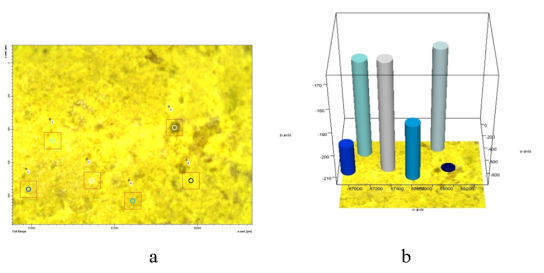
Fig. 1. Microphotography of the catalyst surface (a), its 3D image (b)
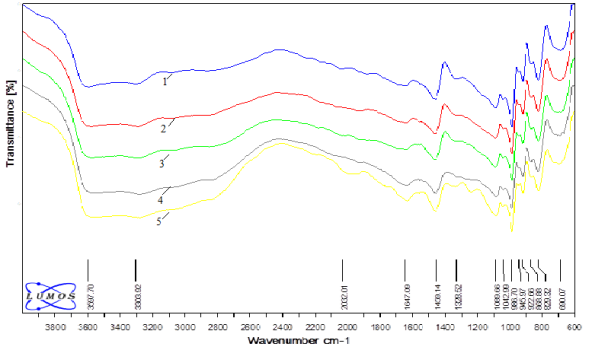
Fig. 2. IR spectra of selected points-1,2,3,4,5 on the surface of the peroxomolybdate catalyst containing Gd shown in Figure 1
The surface morphology of polyoxomolybdate and its peroxomolybdate samples treated with hydrogen peroxide at 40–60 ° C with Gd-containing -Gd n PMo m O 40 (Gd n PW m O 40 ) (where n = 3–6, m = 12–14) is characterized by a surface consisting of 1–3 μm fragments (Figure 3a and Figure 3b). In this case, in contrast to the primary polyoxomolybdate complex, partial amorphization and increase in the dispersion of the crystal structure are observed in the peroxocomplex. The decomposition of the structure of the primary polyoxomolybdate complex when mixed with a solution of hydrogen peroxide is also confirmed in the X-ray phase analysis of their individual samples. Figure 4 describes the mapping of the presented catalyst under a microscope.

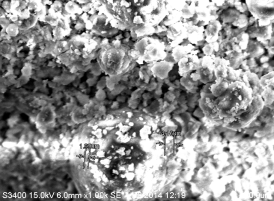
Fig. 3. Microphotography of the GdPO 4 ∙H 2 O∙MoO 2 P 0,5 Mo 14 O 42 system
a) polyoxomolybdate complex; b) peroxomolybdate




Fig. 4. Mapping of elements by GdPO 4 ∙H 2 O∙MoO 2 P 0,5 Mo 14 O 42 microphotography (Mapping)
Calculation of distances according to the dimension 2 in the diffractogram, 2 = 22.3 in the catalyst; 29.8 and 32.0 GdPO 4 H 2 O d = 4.04, 2.978, respectively; 279; 2.63; 2,057; 1.82 indicates that the A phase is predominant. On the diffractogram (MoO 2 ) 0,5 PMo 14 O 42 d=3,30; 6,325; 2,74; Phases of 2.50 A are also observed.
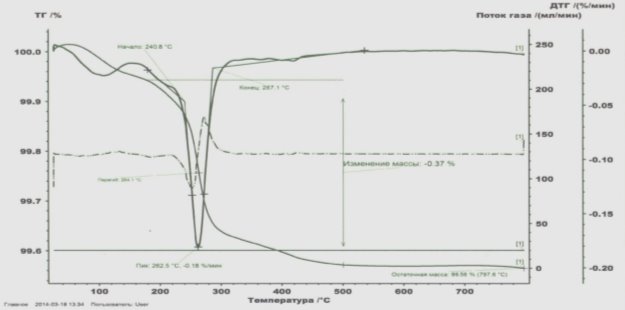
Fig. 5. Thermogravimetric curve of poloxophosphoribolybdate modified by Gd
Only one major endothermic peak (240.8–287.1 ºC) is observed in the thermogravimetric curve of Gd polyoxophosphorolybdate. This is due to the separation of volatile substances in the catalyst. The catalyst is heated to 797.6 ºC and remains almost unchanged at 99.56 % with very little mass loss. This proves that the catalyst is thermostable (Figure 5). Thermogravimetric curves of polyoxo- and peroxocomplexes show that they lose weight in several stages. In the initial stage, exothermic peaks in the range of 22.7–180 ºC are associated with loss of water and peroxide. In the second stage, the loss of mass in the area of 305–610 ° C is due to the conversion of carbon mass into CO 2 . In this case, the black color of the catalyst turns yellow. Heating to 800 ° C causes complete decomposition of the complex and the formation of a mixture of molybdenum and NTE-oxide.
POM have previously been used as modifiers in the preparation of ANdM (Aluminoneodiummolybdate) catalysts for the hydrofinishing of petroleum fractions. For the same purpose, REE additives were used. The possibility of increasing the hydrogenation and hydrodesulfurization of HDS activity of ANM catalysts with the addition of Nd salt in relation to the heavy straight-run diesel fraction and light coking gas oil has been shown. The optimal amount of this additive has been determined. The combined use of neodium and POM, studied in this work, makes it possible to increase the activity of the catalyst in a wide temperature range.
A complex of additives and a combined synthesis method were used to prepare a catalyst with increased HDS activity. All synthesized samples contained the same amount of Mo and Nd.
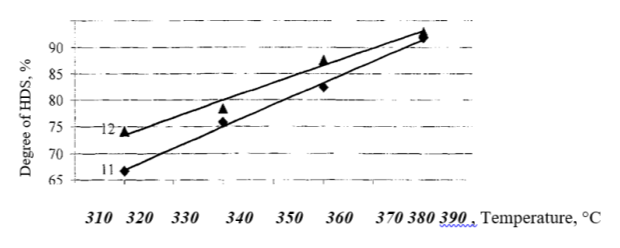
Fig. 6. Comparison of the activities of ANdM and ACoM catalysts
Thus, it has been shown that the introduction of neodium additive increases the activity of ACoM catalysts by 9 % rel. on average, while the sulfur content in the hydrogenation product decreases to 0.002 % wt. (at 380 °C).
The physicochemical properties of the synthesized polyoxometalates have been studied. It has been shown that up to 440°C the structure of the NdPOM is not destroyed and this compound does not decompose at the hydrothermal stage of the catalyst synthesis. For the first time, the synthesis and testing of the catalytic activity of catalysts using REE POM was carried out. It was shown that the catalyst based on NdPOM exhibited the maximum hydrodesulphurization activity. It is shown that the catalyst makes it possible to carry out hydrodesulfurization of mixed feedstock to a residual sulfur content of 0.002 wt %.
References:
- Mitusova T. N., Kalinina M. V. Diesel and biodiesel fuels// Oil refining and petrochemistry. 2004. No. 10. pp.11–14.
- Kashin O. N., Ermolenko A. D., Firsova T. G., Rudin M. G. Problems of production of high-quality gasolines and diesel fuels./ Oil refining and petrochemistry. 2005. No. 5. pp.32–38.
- Krylov I. F., Emelyanov V. E., Nikitina E. A. Low-sulphur diesel fuels: pros and cons.// Chemistry and technology of fuels and oils. 2005. No. 6. S.3–6.
- Loginov S. A., Kapustin V. M., Lugovskoy A. I. and others. Industrial production of high-quality diesel fuels with a sulfur content of 0.0035 and 0.05 %. // Oil refining and petrochemistry. 2001. No. 11. pp.57–61.
- Fedorinov I. A., Anisimov V. I., Moroshkin Yu G. et al. Experience in obtaining ultra-low-sulfur diesel fuels according to the EN 590–2005 standard at Lukoilvolgogradneftepererabotka LLC // Oil refining and petrochemistry. 2006. No. 1. S. 10–12.
- Fuel. Lubricants. Technical liquids. range and application. Handbook, ed. Shkolnikova V. M. M: Chemistry. 1999. 372 p.
- GOST 3900–85. Oil and oil products. Density determination methods.
- Belyanin B. V., Erich V. N., Korsakov V. G. Technical analysis of oil products and gas. L.: Chemistry, 1986. — 184 p.
- Tomina H. N., Loginova A. N., Sharikhina M. A. et al. Method for catalytic upgrading of products of thermal processes. Pat 2147597 RF, MPK7 C 10 G 11/10. No. 98120294/04; dec. 11/11/98; publ. 04/20/2000, B. I. No. 11–7 p.
- Tomina H. N., Loginova A. N., Sharikhina M. A. et al. Catalyst for hydrotreatment of petroleum fractions and method of its preparation. Pat. 2147255 RF, MPK7 V 01 J 23/88, C 10 G 45/08. No. 98105317/04; dec. 03/17/98; Published 01/27/2000, B. I. No. 10–6 p.
- Fomichev Yu.V., Tomina H. N., Loginova A. N. and other Method of preparing a catalyst for the hydrotreatment of petroleum raw materials. Patent of the Russian Federation No. 1491564 Pat 1491564 of the Russian Federation, reg. 01. 10.01. (A.C. 1491564 USSR, MKI4 V 01 J 37/02. No. 4152635/23–04; application 11/26/86; publ. 07/07/89, Bull. No. 25–5 p.)
- Loginova A. N., Tomina M. A., Sharikhina M. A. et al. Method for hydrotreatment of petroleum distillate fractions. Patent of the Russian Federation No. 2030444. Pat. 2030444 Russian Federation, MPK6 C 10 G 45/08. No. 5055449/04; dec. 07/20/92; publ. 10.03.95, B. I. No. 7–4 p.







