Вданной работе рассматривается использование основных методов промышленного получения меллитовой кислоты из различных исходных веществ и процессов производства, имеющие определенные недостатки. В дальнейшем будет найден оптимальный способ получения меллитовой кислоты.
Ключевые слова: меллитовая кислота, методы, оптимальный, промышленность, электролиз.
Mellitic acid is a six-base acid of the aromatic series with the formula C6(COOH)6 (Fig. 1).The study of the properties of aromatic polycarboxylic acids opens up the prospect of their diverse application in the production of synthetic polymers for various applications that have unique properties.
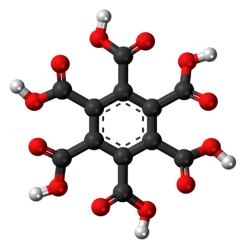
Fig. 1. Structural formula of mellitic acid
Up to 80s of 20th century there was no industrial method of production of mellitic acid, but possibility of wide use of its derivatives attracted attention of chemists, therefore there are enough researches on development of method of its production [1] [3] [4] [7]. Mainly methods based on oxidation of pretreated coal or graphite, including electrochemical process are considered [3] (Fig.2).

Fig. 2. Electrochemical process of pre-treated coal or graphite to produce mellitic acid
The wide use of mellitic acid derivatives is hampered by the difficulty in obtaining them. Currently, the most common way to produce mellitic acid is two-stage oxidation of pre-crushed fossil coals or products of their heat treatment [1] [2] [6] [7].
The main disadvantages of this method are:
multistage oxidation process taking place in acidic, alkaline and neutral media;
high corrosiveness of the nitric acid oxidiser and the need to handle toxic gases — chlorine and nitrogen oxides
need for hazardous handling of hot nitric acid; — extremely low mellitic acid yield (less than 1 g per 5 litres of nitric acid);
the necessity to prepare nitric acid with a concentration of more than 80 % due to a lack of acid with this concentration;
necessity of additional heat treatment of coal at 600–650oC to obtain mellitic acid yield of more than 4.5 %;
The presence of impurities in the target product.
Our hypothesis is that mellitic acid can be to be produced under so-called «mild conditions». The method consisting in electrochemical anodic oxidation of carbon-graphite materials to mellitic acid is the closest to the most rational method with respect to technical essence and the result achieved. The process is carried out by direct oxidative destruction of carbon-graphite anode. [1] [3] [6] [7].
The main disadvantages of this method are:
need to use pre-formed carbon-graphite anode in the process, as the target product is obtained by its direct destruction in the electrolyte;
impossibility of using fossil coal as a raw material for electrochemical oxidation, and similar materials with low electroconductivity, as anodes, formed from them, practically are not subject to electrochemical oxidation;
the duration of the electrochemical process as only the exposed surface of the carbon-graphite anode in the electrolyte is oxidised;
the multistage and difficulty of extracting the target product;
use of corrosive hot sulphuric acid solution as an electrolyte .
In this work we carried out the electrochemical oxidation of fossil coals and graphite; the oxidant used was the less corrosive 'active chlorine' produced by the electrolysis of sodium chloride solution (Fig.2). The term «active chlorine» refers to the total content of hypochlorite ions in the solution ClO-, hypochlorous acid HClO and free chlorine Cl2.
We considered the result of the reaction to be 1.1 g of polycarboxylic acid, of which 10 mg is mellitic acid [4] [5] [7]. An indisputable advantage of the above reaction is that the process is periodic — the reaction can be interrupted and restarted at any time without any losses.
The advantage of this method is:
electrochemical oxidation of fossil coal or graphite occurs in the whole volume of the electrolyte, which eliminates the need for preliminary formation of the anode for its oxidation according to the prototype;
the possibility of using as a readily available raw material for the oxidation of fossil coals with low electrical conductivity;
use of «active chlorine» as an oxidizer,
produced by the electrolysis process, which makes the process safer and more environmentally friendly.
The process of extracting the target product from the electrolyte after the oxidation process is considerably simplified, and the electrolyte can be used again after neutralisation for the next electrochemical oxidation cycle.
The electrolyte can be used again after neutralisation for a further electrochemical oxidation cycle;
The oxidant «active chlorine» generated electrochemically in sodium chloride solution is converted into the same salt by oxidising the fossil carbon or graphite.
Thus, the oxidation process of coal is cyclic [3] [7].
References:
- Galikeev A. R., Galiamov E. Z. Method of benzene-hexacarboxylic acid (mellitic acid)/Patent RF2098402, patent holder: Ufa State Petroleum Technical University, publication of patent: 10.12.1997
- Golubeva T. B., Tselischeva S. V. Catalytic systems in chemistry course. / Manuals for laboratory and practical classes for students of full-time and correspondence forms of education. — Ekaterinburg, Ural State University, 2011. -12с.
- Shapkin I. P., Polyakov V. Y., Kondrikov N. B. Method of benzene-polycarboxylic acids preparation / Patent RF2194034, patent holder: Far Eastern State University, publication patents: 10.12.2002
- «An inexpensive and efficient synthetic method for the preparation of pyromellitic dianhydride promoted by ionic liquid» Yu Lin Hu, Ming Lu, Xiao Bin Liu, Sheng Bin Zhang, Zhan Hui Ji, and Ting Ting Lu, Chemical Engineering College, Nanjing University of Science and Technology, Nanjing 210094, PR China.
- The new chemist's and technologist's handbook. General properties of Inorganic, organic and organometallic compounds. — S.-Pb.: ANO NGO «Peace and Family», 2002. — 1280p.
- Zinnurova O. V., Fattakhov D. A. The main methods of mellitic acid production and their disadvantages. / Young Scientist. — 2022. — № 13 (408). — p. 83–85. (URL: https://moluch.ru/archive/408/89946/ (date of reference: 03.06.2022).
- Fattakhov D. A., Zinnurova O. V. Study of the optimum method for producing mellitic acid. / Living in the 21st century — 2022. — p. 81–85.







