Nitramine compounds (NAs) such as hexogen (xycltrimethylenepinitramine, RDX), octogen (xyltetramethylenetetramitramine, HMX), and tetryl (2,4,6-trinitrophenyl-N-methylnitramine, TET) are commonly used as strong explosives. They are commonly found in contaminated wastewater from some industrial explosives production lines, such as for ammunition or rocket propellants. For instance, tetryl is employed in explosive charges, and in gunpowder. RDX and HMX on the other hand are commonly used in bombs, torpedoes, and armor-piercing shells. As nitramine compounds cause harm to human health, animals and ecosystems, such contaminated water has already been subject to earlier research
In recent years, to treat the nitramine compounds contaminated water has been tested using a variety of methods such as hydrolysis [5], electrochemical [6], microorganism [7–10], chemical advanced oxidation processes or electro-chemical advanced oxidation processes. However, there are especially limitations on compounds as RDX, HMT, TET when degradation of nitramine compounds use AOPs showed the speed and efficiency of decomposition of nitramine compounds. To date, there has been a lack of publicity regarding the degradation characteristics in water by photo-Fenton. According to the published research, there are little attention about the comparison and evaluation of the conversion efficiency of UV radiation and UV-H 2 O 2 or UV-Fenton.
The purpose of this study is to present the results of the survey, comparison and evaluation about influence of UV radiation and the effect of UV-H 2 O 2 and photo-Fenton on metabolic efficiency as well as on dynamic characteristics of the NAs degradation such as RDX, HMX and Tetryl.
Materials and methods
Materials: Crystalline RDX (99 %), HMX and Tetryl were of analytical grade. All solvents (acetonitrile, ethanol, methanol and hexane) used in experiments were of HPLC grade. FeSO4.7H2O, hydrogen peroxide (30 %) and all other reagents were of analytical grade. All chemicals were purchased from Merk.
Instruments: Tetryl, RDX and HMX were identified and quantified by an HPLC (HP Agilent 1100 series, diode array detector, USA), using a Hypersil C18 column (200 mm and 4 mm). The pH of the solution was adjusted by adding aqueous H 2 SO 4 or NaOH to the desired value, determined by a pH meter (OAKLON, 510 series, USA).
Setup experiments: All experiments were carried out in a home-built circulating photoreactor system. A detailed set-up of this system can be found elsewhere [13,14]. The reaction solution was introduced by adding NAs and an oxidative agent (i.e. H 2 O 2 or Fenton’s reagent) into the reservoir equipped with a temperature controller. The initial pH of the solution was adjusted to pH 3 with H 2 SO 4 . Solution was stirred at a speed of 300 rpm during the experiment and circulated through a UV reaction chamber using a metering pump at a flow rate of 700 mL/min. Samples were withdrawn from the solution at 5- min intervals in order to analyze the remaining nitramin explosives content and reaction intermediates by HPLC. In the processes combined with irradiation, a 15 W UV– light lamp (radiation wavelength 254 nm) located at the reaction chamber core was turned on during the reaction
Analytical methods: Tetryl, RDX and HMX were identified and quantified by an HPLC (HP Agilent 1100 series, diode array detector, USA), using a Hypersil C18 column (200 mm 4 mm). The mobile phase consisted of 67 % acetonitrile and 33 % water (v/v) at a flow rate of 0.6 mL min1, with a pressure of 280 bar. The analytical signal was measured at the wavelength of 227 nm. HPLC peaks of Tetryl, RDX, HMX were observed at retention times (tR) 5.7min, 5.11 min and 4.7min HMX, respectively. The amount of 3 explosives was quantified using the external standard method.
The conversion (η, %) of nitramin explosives in photo-Fenton systems is calculated by the formula:
η = {(C (0) — C (t) )/C (0) }x100 (1)
Where is the conversion of nitramine explosives (%), C (0) : concentration of RDX at time t= 0; C (t) : concentration of nitramine explosives at time t.
Results and discussion
In Figure 3.1, the curves showing the conversion results (η, %) of some of the NAs compound by reaction time in the chemical oxidation system with only one photocatalyst (NAs/UV), or an oxidizing agent is H 2 O 2 (NAs/H 2 O 2 ) and CAOPs based on OH radicals derived from the Fenton effect (NAs/Fenton); the UV-H 2 O 2 effect (NAs/UV-H 2 O 2 ) and UV-Fenton effect (NAs /UV-Fenton)
a)
b)
c)
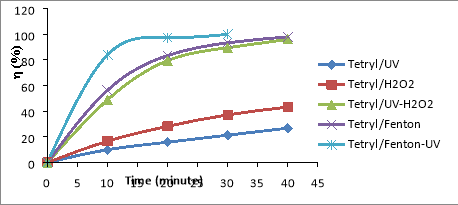
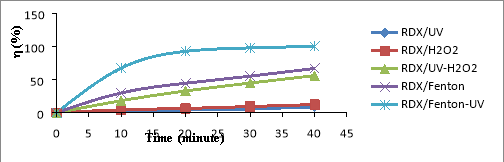
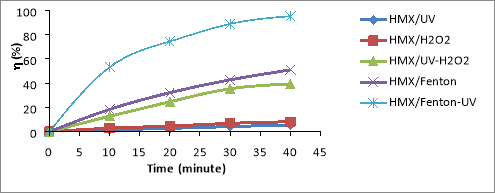
Fig. 1. Relationship (%)-t for RDX (a), HMX (b) and Tet (c) in 5 CAOP systems: NAs/UV; NAs/H 2 O 2 ; NAs/UV-H 2 O 2 ; NAs/Fenton; NAs/UV-Fenton: T=25 o C, C Tet = 38.46 mg/L, C RDX = 19.22 mg/L, C HMX = 5.28 mg/L, C H2O2 = 14.5 mM, C Fe2 += 0,675 mM, I = 875 Lux, pH = 6
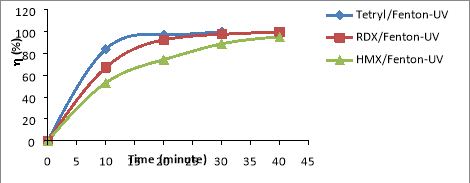
Fig. 2. Comparing RDX, HMX and Tet decomposition efficiency in the same NAs/Fenton-UV system: C Tet = 38.46 mg/L, C RDX = 19.22 mg/L, C HMX = 5.8 mg/L, C H2O2 = 14.5 mM, C Fe2 += 0.675 mM, I = 875 Lux, pH = 6
The results shown in Figures 1a-1c, it is found that the decomposition efficiency of NAs compounds (
NAs
%) in the CAOPs investigated ascending the ranges: NAs/UV< NAs/H
2
O
2
< NAs/Fenton< NAs/NAs/UV-H
2
O
2
From the results shown in Figure 3.2, the NAs decomposition efficiency increases with the range HMX< RDX< Tet. Thus the law of NAs transformation in CAOPs is similar to that of NPs for NPs (especially trinitrophenols (TNPs) [13], or compounds ester nitrate (ENs) [14].
In the NAs/UV system, NAs decay efficiency is generally very low because UV has only low quantum efficiency [15]. The direct decomposition effect of UV is only promoted in the case of unsustainable organic compounds. The degradation efficiency of NAs can increased when the NAs solution is supplemented with a certain amount of H 2 O 2 with a concentration of C H2O2 greater than 5mM. This H 2 O 2 may be involved in the direct oxidation of NAs, although this reaction is not high effect because H 2 O 2 is lower oxidative activity than OH [16]. In the system of UV-H 2 O 2 (as NAs/UV-H 2 O 2 ), a direct UV photocatalytic reaction of H 2 O 2 [17]:
H 2 O 2 + hv 2 OH (3.1)
The oxidation of OH is much higher than H 2 O 2 (E ox( OH) =2.8V, E ox(H2O2) = 1.78V) [16], so the metabolism efficiency of NAs in the system NAs/UV-H 2 O 2 is significantly larger than the NAs/H 2 O 2 system. So in the non-Fenton systems (no reaction between Fe 2+ and H 2 O 2 ), OH- the product of the UV-H 2 O 2 effect is always the most potent oxidizer to effect on speed and efficiency of NAs degradation in the surveyed oxidation systems.
The main effect of UV-H 2 O 2 system has been to increase the amount of OH involved in the oxidation of NAs. In addition, in the reaction systems based on this effect, apart from the main effect of OH, it is necessary to take into account the simultaneous effects of UV radiation even though the direct quantum photonic activity is not high [15].The influence of UV makes bonds in the NAs molecule more weakly, more flexibly and therefore it is more susceptible to attack by free radicals OH. Thus, it can be said that the UV-H 2 O 2 effect has the potential to produce a double-acting (simultaneous action of both OH and UV) on the metabolism of NAs as well as other organic compounds. This is always greater than the effects of individual oxidizing agents such as H 2 O 2 , OH or UV.
In the NAs/Fenton system, NAs are primarily decomposed by OH radicals produced by the Fenton reaction between H 2 O 2 and Fe 2+ .
Fe 2+ + H 2 O 2 OH + OH - + Fe 3+ (3)
However, the Fe 2+ and OH quantities were reduced during the reaction, so the decomposition rate of NAs remained unstably and diminished over time. This phenomenon will be overcome when using the optical Fenton effect (for example, the NAs /UV-Fenton system). In this system, the OH is generated simultaneously from three reactions: the usual Fenton reaction between Fe 2+ with H 2 O 2 (reaction 3), UV-H 2 O 2 reaction (reaction 2) and the photosynthetic reaction [Fe 3+ (OH) - ] 2+ by UV at pH <4 (reaction 4) [16,18,19].
[Fe 3+ (OH) - ] 2+ + hv OH + Fe 2+ (4)
By these reactions, there are much more OH involved in the reaction, continuously regenerating the Fe 2+ catalyst.
Especially the complex of [Fe 3+ (OH) ] 2+ that absorbs photons much stronger than H 2 O 2 at wavelength 254 nm, so the photosynthetic reaction [Fe 3+ (OH) ] 2+ is much greater than the H 2 O 2 photosynthesis in the formation of OH radicals. This is also the reason which the NAs of the NAs/UV-Fenton is larger than the NAs of the NAs/Fenton, the NAs/UV-H 2 O 2 and the NAs/EO-UV-H 2 O 2 systems.
Conclusion
The UV radiation and effects such as UV-H 2 O 2 or UV-Fenton have important influences on the efficiency and rate of decomposition of nitramine compounds such as RDX, HMX and Tetryl by the advanced oxidation processes and the electrochemcal oxidation processes. In all of the chemical oxidation and the electrochemcal oxidation processes have been investigated that process with UV-H 2 O 2 , or UV-Fenton effect has the high NAs decomposition rates and the high NAs decomposition efficience. The direct effect of UV radiation at the same time as the active of the OH radicals which are the product of the UV-H 2 O 2 or UV-Fenton effect to be the main cause of this phenomenon.
The survey results show that by using UV radiation and UV-H 2 O 2 effects, UV-Fenton can significantly improve the efficiency of chemical oxidation and especially electrochemical. They can treat and clean contaminated water sources, such as nitramin compounds.
References:
- Kyung-Duk Zoha, Michael K. Stenstromb Fenton oxidation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) Water Research 36 (2002) 1331–1341
- Burton DT, Turley SD, Peters GT. The toxicity of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) to the freshwater green alga selenastrum-capricornutum. Water Air Soil Pollut 1994;76:449–57.
- McLellan W, Hartley WR, Brower M. Health Advisory for Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine;Technical Report No. PB90–273525; Office of Drinking Water, US. Environmental Protection Agency: Washington, DC, 1988.
- International Journal of Toxicology,V.18,No.2,97–107 (1999). Toxicity of Tetryl (N-Methyl-N,2,4,6-Tetranitroanilin) in F344 Rats
- Heilmann H, Wiesmann U, Stenstrom MK. Kinetics of the alkaline hydrolysis high explosives RDX and HMX in aqueous solution and adsorption to activated carbon.Environ Sci Tech 1996;30(5):1485–92.
- Do Ngoc Khue a, *, Tran Dai Lam b, *, Nguyen Van Chat a, Vu Quang Bach a, Do Binh Minh a, Vu Duc Loi c, Nguyen Van Anh. Simultaneous degradation of 2,4,6-trinitrophenyl-N-methylnitramine (Tetryl) and hexahydro-1,3,5-trinitro-1,3,5 triazine (RDX) in polluted wastewater using some advanced oxidation processes. Journal of Industrial and Engineering Chemistry 20 (2014) 1468–1475
- Freedman DL, Sutherland KW. Biodegradation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) under nitratereducing conditions. Wat Sci Technol 1998;38(7):33–40.
- Coleman NV, Nelson DR, Duxbury T. Aerobic biodegradation of Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biol Biochem 1998;30(8–9):1159–67.
- Ronen Z, Brenner A, Abeliovich A. Biodegradation of RDX contaminated wastes in a nitrogen-deficient environment.Wat Sci Tech 1998;38(4–5):219–24.
- Zoh KD, Daniels JI, Knezovich JP, Stenstrom MK.Treatment of hydrolysates of the high explosives Hexahydro-1,3,5-Trinitro-1,3,5-Triazine and Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine using biological denitrification. Water Environ Res 1999;71(2):148–55.
- Bose P, Glaze WH, Maddox DS. Degradation of RDX by various advanced oxidation processes: i. Reaction rates. Wat Res 1998;32(4):997–1004.
- Bose P, Glaze WH, Maddox DS. Degradation of RDX by various advanced oxidation processes: ii. Organic byproducts. Wat Res 1998;32(4):1005–18.
- Do Ngoc Khue, Tran Dai Lam, Do Binh Minh, Vu Duc Loi, Nguyen Hoai Nam, Vu Quang Bach, Nguyen Van Anh, Nguyen Van Hoang and Dao Duy Hung Enhancement of Electron Transfer in Various Photo-Assisted Oxidation Processes for Nitro-Phenolic Compound Conversion. Journal of Electrolic Materials.
- Do Ngoc Khue*, Tran Dai Lam, Dao Duy Hung, Vu Quang Bach, Nguyen Van Anh, Nguyen Hoai Nam, Nguyen Viet Thai and Do Binh Minh Parameters controlling the advanced oxidation degradation kinetics of nitroglycerin and pentaerythritol tetranitrate. Journal: Green Processing and Synthesis Article-
- J. G. Calvert and J. N. Pitts, Photochemistry (New YorkLondon-Sydney: Wiley, 1966
- H. Zhou and D. W. Smith, J. Environ. Eng. Sci. 1, 247 (2002).
- G. Ruppert, R. Bauer, and G. Heisler, J. Photochem. Photobiol. A 73, 75 (1993)]







