The study isolated purple non-sulfur bacteria from water samples of shrimpfarming at Truong Dinh Village, Hoa Lien Commune, Hoa Vang District, Da Nang City, and Hiep Hai Village, Binh Hung Commune, Thang Binh District, Quang Nam Province. The results showed that one strain of RH02 (Rhodobacter capsulatus) bacteria was isolated and identified. Determining the optimal culture conditions for this strain is the DSMZ — 27 habitat at 28 -30°C, pH = 7, salinity 25‰, and biomass collection time after 72h of culture. The Rhodobacter capsulatus strain processed organic compounds after seven days of testing, with an 80–85 % efficiency.
Keyword: non-sulfur purple bacteria, shrimp farming, waste water, nitrogen, Rhodobacter capsulatus.
1. Introduction
Annually, the shrimp industry contributes about 40–45 % of total seafood export value, equivalent to 3.5–4 billion USD (Research Institute for Aquaculture No.1, 2013). Besides that, Shrimp farms also need more planning in intensive farming, a large amount of excess feed and organic matter released into the environment, and high-density shrimp farming in chemical treatment. These organic compounds stimulate the growth of microorganisms, pollute ponds, and unbalance the ecosystem (Research Institute for Aquaculture No.1, 2013). Research results show that 48.0–87.3 % (N) and 75.0–94.0 % (P) inputs in shrimp ponds are not absorbed to create shrimp biomass but are released into the environment through water change, discharge when harvesting, and deposition in the bottom mud of the pond. Thus, for each ton of shrimp farming, about 16.8–157.2 kg N and 2.3–45.9 kg P will be released into the environment (Luo et al., 2012).
Among the microorganisms involved in the aquarium's carbon, nitrogen, and sulfur cycles, purple non-sulfur bacteria play an important role in improving water quality. Purple non-sulfur bacteria can grow by photoautotrophs, photoheterotrophs, or heterotrophs, depending on light, oxygen, and suitable carbon sources. They can utilize organic matter with or without sunlight, and some species can remove H2S (Kornochalert et al., 2014.
To contribute to exploiting the potential application of indigenous microorganism’s resources, which are abundant for wastewater treatment of shrimp ponds, we selected a strain of indigenous Rhodobacter sp. ability to process organic compounds.
2. Research methods
2.1. Isolation method of purple photosynthetic bacteria
Water samples were collected in shrimp ponds in Truong Dinh Village, Hoa Lien Commune, Hoa Vang District, Da Nang City, and Hiep Hai Village, Binh Hung Commune, Thang Binh District, Quang Nam Province. The samples were enriched by cultured in glass flasks of 100 ml volume. Wastewater samples were put into bottles at a ratio of 1:1. Then these flasks were filled with DSMZ-27 medium, in non-aerated conditions, under natural light, at a temperature between 28–30 °C, pH from 6.5–7.5. After about a week, colored lines from yellow-brown to burgundy appeared on the jar's walls (representing the presence of pigment characteristic of photosynthetic purple bacteria). Sampling from the colored stripe and then isolated on a petri dish containing DSMZ 27 agar. After about 3–5 days, round brown, pink, and purple colonies appeared. These colonies were cleaned by the streak plate method and incubated under light conditions (Do Thi Lien, 2016).
2.2. Research method of cell morphology, physiological and biochemical characteristics of isolates
After seven days of culture on DSMZ — 27 medium, bacterial colonies were observed under the microscope to record the shape, size, and color of colonies. Carrying out Gram staining, testing the ability to ferment sugar, catalase, degradation of Urea, and Citrate (Bergey's, 1989).
2.3. Identification method of bacterial by molecular biology techniques
Checking isolated Rhdobacter sp. by PCR (Polymerase Chain Reaction)
Primer sequences were used to amplify the 16S rRNA gene region
|
Primer |
Sequences |
|
|
27F |
5’- AGAGTTTGATCCTGGCTCAG — 3’ |
|
|
1492R |
5’ — GGTTACCTTGTTACGACTT — 3’ |
PCR products after amplification were sequenced using automated sequencing. After sequencing the 16S rRNA gene, compare the sequence with GenBank published in the NCBI data bank using the BLAST tool to identify the purple photosynthetic bacteria on the similarity of the 16S rRNA gene sequence with the sequences available on the database.
2.4. Survey method for some factors affecting the growth ability of identified bacterial
a) Effect of pH: Prepare the growth medium DSMZ-27 in 100 ml/L cylindrical glass bottles to survey the pH range 4; 4.5; 5; 5.5; 6; 6.5; 7; 7.5; 8; 8.5; 9.
b) Effect of salt concentration : Prepare the DSMZ-27 medium; the selected strains are grown in 300 ml cylindrical glass flasks, and the salt concentration is investigated in the 0‰ range; ten‰; 15‰; 20‰; 25‰; 30‰; 35‰ and the pH was selected in ''a'' item.
The experiment was conducted under lighting conditions, and record the results after seven days.
2.5. Method of building the growth curve of the identified purple photosynthetic bacteria
Construct a correlation curve between OD and log cell density (CFU/ml)
The 7-day culture broth was diluted in DSZM-27 medium to obtain OD660 nm concentrations of 0.1; 0.2; 0.3; 0.4; 0.5; 0.6, respectively. Then proceed to dilute to 10–8 and inoculate on a plate of DSMZ-27 agar at a concentration of 10–6; 10–7; 10–8.
Build growth curve: Measure the OD value every 12 hours from the culture broth using a Jasco V750 spectrometer. Based on the results of the OD value and the correlation equation to build the growth curve.
2.6. Method to evaluate the efficiency of organic compounds treatment of the identified purple photosynthetic bacteria
Create a hypothetical wastewater environment by adding glucose to have organic concentrations of 10, 50, 100, 200, and 400 mgC/, respectively.
The experiment used 30 cylinders with a volume of 300 ml, added to each flask 2 % bacterial solution obtained from section 2.2.6 with pH and salinity determined in section 2.2.8, adding organic matter content is: 10, 50, 100, 200, and 400 mgC/L, respectively.
Controlled experiment: the environment has salinity and pH determined in item 2.2.8 with organic matter content of 10, 50, 100, 200, and 400 mgC/L, respectively, but without adding Rhodobacter.
The density of Rhodobacter was determined through the cell suspension absorbance on the spectrophotometer. The organic matter content was monitored daily through the permanganate method's COD indicator (QCVN 6186–1996).
2.7. Data processing methods
The experiments were repeated three times. The results are calculated and processed by statistics on MS programs — Excel and Rstudio.
3. Research results and discussion
3.1. Isolation of Rhodobacter sp. from shrimp wastewater samples
From water samples collected in Truong Dinh Village, Hoa Lien Commune, Hoa Vang District, Da Nang City, and in Hiep Hung Village, Binh Hai Commune, Thang Binh District, Quang Nam Province, four strains of bacteria were isolated. Symboled bacteria (RH01 → RH04) could grow in liquid DSMZ — 27 medium (Table 1, Figure 1). The two strains of RH02 have round, convex, glossy, spherical, chain-forming cells, are positive for sugars, citrate, and urea, are negative for catalase, and are gram-negative bacteria, which is consistent with the described characteristics of Rhodobacter sp. according to Bergey's taxonomy and studies by other scientists on this bacterium (Bergey's, 1989; Brenner et al., 2005; Weaver et al., 1975).
Table 1
Colony morphology characteristics of 4 isolated bacteria
|
Name |
RH01 |
RH02 |
RH03 |
RH04 |
|
|
Colony characteristics |
Color |
Dark Pink, Diameter 3,3µm |
Light pink, Diameter 3,1 µm |
Light pink, Diameter 2,6µm |
Light pink, Diameter 1.9µm |
|
Morphology |
Round, wet, convex surface, slimy |
Round, convex surface, glossy |
Round, convex surface, flat edge |
Round, flat surface, inner edge |
|
|
Cell characteristics |
Shape |
Egg-shaped |
Spherical |
Egg-shaped |
Egg-shaped |
|
Gram |
- |
- |
- |
- |
|
|
Biochemical characteristics |
Glucose |
- |
+ |
- |
- |
|
Frutose |
- |
+ |
- |
- |
|
|
Mantose |
- |
+ |
- |
- |
|
|
Catalaste |
+ |
- |
+ |
+ |
|
|
Urea |
- |
- |
+ |
+ |
|
|
Citrat |
- |
+ |
- |
+ |
|
|
Note: (+) positive (-) negative |
|||||
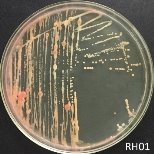
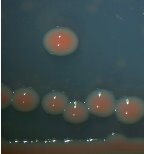
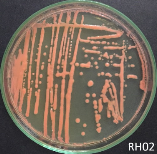
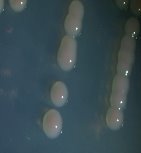
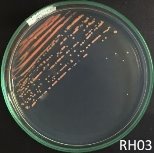

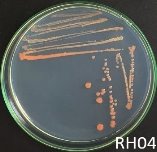
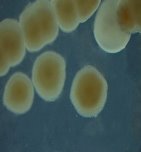
Fig. 1. Colony shapes of 4 strains isolated from wastewater samples
3.2. Identification of rh02 bacterial by molecular biology techniques
RH02 bacteria were identified using molecular biology techniques. The results of reading 16s — rRNA sequences were compared to the NCBI gene bank to identify RH02 species. RH02 bacteria has a sequence of 16s — rRNA gene region, which is 97.70 % similar to Rhodobacter capsulatus species (Figure 2)- allowing the conclusion that RH02 bacteria is a species of Rhodobacter capsulatus.
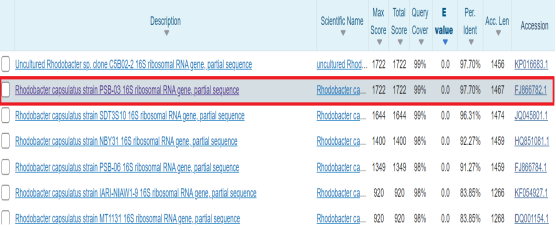
Fig. 2. Searching for homology sequence of Rhodobacter
3.3. Investigate some factors affecting the growth and development of Rhodobacter capsulatus
3.3.1. pH effects
The results of the investigation of pH affection on the growth and development of Rhodobacter capsulatus are shown in Figures 3 and 4.
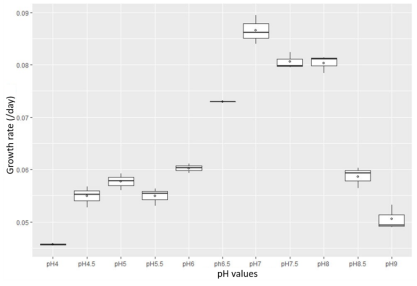
Fig. 3. Growth rate of Rhodobacter capsulatus under light conditions
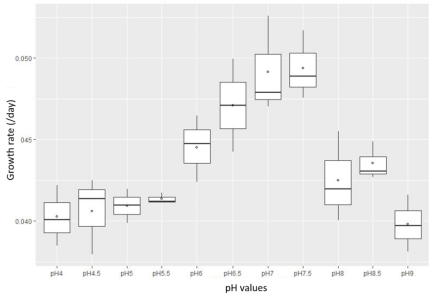
Fig. 4. Growth rate of Rhodobacter capsulatus under shading conditions
The results show that the bacterial strain can grow quite well in the pH range of 6–7.5 in both light and dark conditions. Besides, under pH conditions in which less than five microorganisms are inhibited and have a prolonged growth rate and under light conditions, the results are more optimistic than in dark conditions. The experimental results are consistent with domestic and international studies (Brenner et al., 2005; Do Thi Lien, 2016).
Under light conditions, the growth rate of Rhodobacter capsulatus was faster than in the dark states. The growth rate was maximum at 0.08 with a cell density of 15x108; in dark conditions, the growth rate was packed at 0.08 with a cell density of 15x108 and peaked at only 0.05 with a cell density of 12x108. Rhodobacter capsulatus is a microorganism that can conduct photosynthesis to collect and receive energy to serve the living activities of the cell (CA, 1982). Therefore, growth and development are faster in light conditions than in dark conditions.
To apply the isolated Rhodobacter capsulatus strain to test the ability to treat organic compounds in water, pH = 7 was selected.
3.3.2.Salinity effects
The survey results in Figures 5 and 6 show that the Rhodobacter capsulatus strain can adapt to an environment with salinity up to 35 ‰. Still, at 25 salinity, the growth rate is the best.
Besides, when comparing the growth rate with light and dark conditions, it was found that this bacterial strain grew better in lighting conditions nine times.
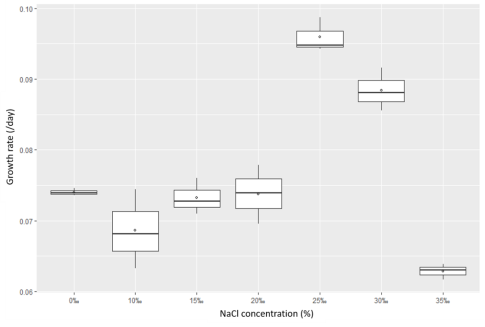
Fig. 5. The growth rate of Rhodobacter capsulatus (NaCl was added to determine the concentration 0‰; 10‰; 15‰; 20‰; 25‰; 30‰; 35‰, under light conditions) C
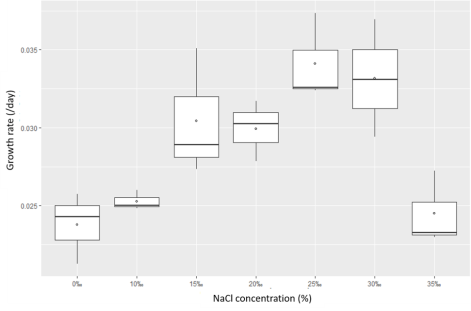
Fig. 6. The growth rate of Rhodobacter capsulatus (NaCl was added to determine the concentration 0‰; 10‰; 15‰; 20‰; 25‰; 30‰; 35‰, under shading conditions)
The results above showed that the Rhodobacter capsulatus bacteria isolated from shrimp wastewater is salt tolerant. This research result is consistent with the study of Bergey and Tra, 2015; Brenner et al., 2005).
3.4. Survey on the growth curve of the Rhodobacter capsulatus
The growth and development of Rhodobacter capsulatus are shown by the growth curve and growth rate. The results are presented in Figure 7.
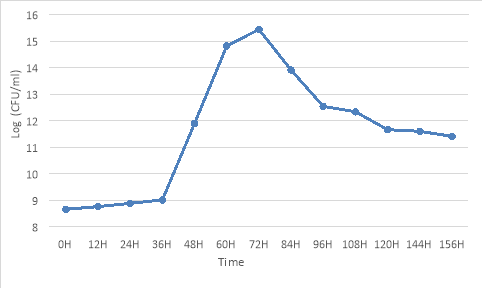
Fig. 7 Growth rate of Rhodobacter capsulatus
The results showed that Rhodobacter capsulatus had a latent phase lasting about 36h. The exponential phase lasted 24 to 36 hours, corresponding to a maximum growth rate of 0.04 at 72h of culture (15x10 8 CFU/ml). However, as the culture time continues to be prolonged, the cell density gradually decreases and begins to decline due to the depletion of nutrients in the medium; the competition for nutrients by bacteria takes place, together with the products of metabolism inhibiting the growth of bacteria, the number of cells produced is less than the number of cells lost (Costa et al., 2017).
3.5 . Evaluating the efficiency of organic compounds processing ability of Rhodobacter capsulatus.
To evaluate the ability of organic matter treatment of the Rhodobacter capsulatus , an experiment was carried out to grow this strain on wastewater with the assumption of adding glucose to the carbon content of 10 mg/L; 50 mg/L; 100 mg/L; 200 mg/L; 400 mg/L, with salinity 25‰ and pH=7. The experiment was conducted under lighting conditions of 3000 lux. Their biomass accumulation and organic matter treatment were monitored for seven days. The results are shown in Figure 8, Figure 9, and Table 2.
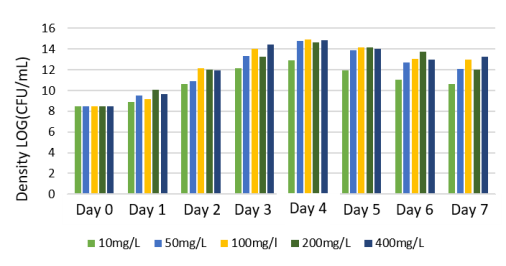
Fig. 8. Growth ability of Rhodobacter capsulatus after seven days
The results from Figure 9 show that the isolated Rhodobacter capsulatus could grow at almost all carbon concentrations from 10 mg — 400 mg/L. The content 50mg/L, 100mg/L, 200mg/L, and 400mg/L had better growth rates and ability than those at 10mg/L concentration. The ability to accumulate biomass at concentrations of 50; 100; 200; 400mg/L gave similar results, and both peaked at day 4 with a density of ~ 15x108 (CFU/ml). Meanwhile, at the concentration of 10mg/L, the growth rate was lower; the highest density was only 12x10 8 (CFU/ml) on day 4.
Table 2
V ariation of organic matter content and treatment efficiency after seven days of testing
|
Carbon content |
Bacterial |
Non-bacterial |
||||
|
COD content on Day 0 |
COD content on Day 7 |
Carbon removal efficiency (%) |
COD content on Day 0 |
COD content on Day 7 |
Carbon removal efficiency (%) |
|
|
10 mg/L |
65 |
36 |
45 % |
65 |
50 |
16.60 % |
|
50 mg/L |
176 |
25 |
85.80 % |
176 |
126 |
28.40 % |
|
100 mg/L |
315 |
40 |
87.30 % |
315 |
280 |
11.10 % |
|
200 mg/L |
700 |
76 |
89 % |
700 |
650 |
7.14 % |
|
400 mg/L |
1426 |
219 |
84.60 % |
1426 |
1024 |
28.10 % |
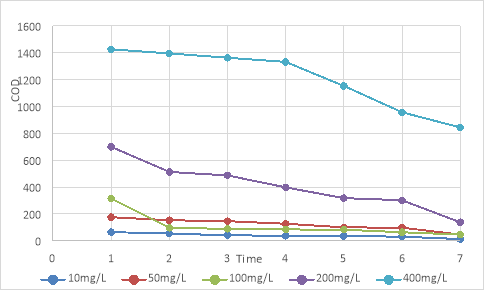
Fig. 9. The ability of Rhobacter capsulatus to remove COD after seven days
The results from Tables 2 and 9 show that Rhodobacter capsulatus can process organic compounds at relatively high concentrations. In the survey treatments, the ability to treat the best results at concentrations of 50; 100; 200; 400 mg/L has an efficiency of 80–85 %, while at a low concentration of 10 mg/L, the treatment efficiency is only 45 %, through that the higher the organic matter content, the higher the growth and treatment efficiency of the strain. It shows that the isolated Rhodobacter capsulatus bacteria can decompose organic substances and use them as a source of substrates for their life activities. This result is entirely consistent with My Tran Huong Tra et al. and the study of Costa et al. (Tra et al., 2015; Costa et al., 2017).
4. Results
Isolation and identification of an RH02 bacteria ( Rhodobacter capsulatus ) from wastewater samples from shrimp ponds in Hiep Hung Village, Binh Hai Commune, Thang Binh District, Quang Nam Province. Determining the optimal culture conditions for this strain are DSMZ — 27 medium at 28 -300C, pH = 7, salinity 25‰, and biomass collection time after 72h of culture. Investigated the ability to process organic compounds of the Rhodobacter capsulatus and found that after seven days of testing, the bacteria could process organic compounds at concentrations of 50; 100; 200; 400 mg/L with an efficiency of 80–85 %.
References:
- Bergey’s. (2001). Mycoplasma. In Infectious Diseases in Obstetrics and Gynecology, Sixth Edition.
- Brenner, D. J., Krieg, R., Staley, J. T., & Garrity, G. M. (2005). Manual® of Systematic Bacteriology: Volume Two The Proteobacteria Part C The Alpha-, Beta-, Delta-, and Epsilonproteobacteria. In Bergey’s Manual of Bacterial Systematics (Vol. 2, Issue 2).
- CA, W. (1982). Dynamics of photosynthetic membrane composition and function. Wraight CA (1982) Current Researchs on Photosynthesis, In Godvindjee (Ed), Photosynthesis, Vol.I, Academic Press. New York, London., 1058(2), 87–106.
- Costa, S., Ganzerli, S., Rugiero, I., Pellizzari, S., Pedrini, P., & Tamburini, E. (2017). Potential of Rhodobacter capsulatus grown in anaerobic-light or aerobic-dark conditions as bioremediation agent for biological wastewater treatments. Water (Switzerland), 9(2).
- Do Thi Lien. (2016). Research on the application of purple photosynthetic bacteria to treat Sulfide in polluted water sources. 1–25.
- N., Kantachote D., Chaiprapat. S. (2014). Use of Rhodopseudomonas palustris P1 stimulated growth by fermented pineapple extract to treat latex rubber sheet wastewater to obtain single cell protein. Annals of Microbiology, 64, 1021–1032.
- Tra, M. T. H. (2015). Research on the culture and use of Rhodobacteria to treat organic matter and sulfide in polluted water on a laboratory scale
- Research Institute for Aquaculture N 1 (2013). Project: Controlling environmental pollution in aquaculture (Shrimp, Pangasius) until 2020. 2020, 36.
- Wang, J., Nie, X., Zhang, L., & Zhao, Z. (2016). Optimization of production procedures for coenzyme Q 10 from Rhodobacter sphaeroides. 8(7), 924–929.
- Weaver, P. F., Wall, J. D., & Gest, H. (1975). Characterization of Rhodopseudomonas capsulata. Archives of Microbiology, 105(1), 207–216.







