Persulfate (PS) is activated by nano zero valent iron without and with UV lights that produces free radicals ( • OH, SO 4 −• ) in the water environment. These radicals decompose organic matter in the water. Investigation of decomposition kinetics of methyl orange (MO) in the activated persulfate systems by nano-zero valent iron (ZVI) without UV lights: nZVI/PS/MO; with UV lights: nZVI/PS/MO/UV. The results showed that the decomposition of MO in the activated persulfate systems obeys the first pseudo order kinetics. The first order apparent reaction rate constants in these systems were calculated. The decomposition efficiency of MO in the systems with UV lights was higher than that in the systems without UV lights.
Keywords : activated persulfate, hydroxyl radical, sulfate radical, azo dye, nano zero valent iron, UV.
Advanced oxidation processes (AOPs) have been studied and applied to treat wastewater and contaminated groundwater in the world. The AOPs is based on the in-situ free radicals which are generated in reaction. These free radicals have high oxidation activity like hydroxyl radicals OH (E = 2.8V) and sulfate radicals SO 4 (E = 2.6V). The free radicals selectively react to all most organic compounds in the water, decomposing and converting the organic compounds into non-toxic or less toxic substances to humans and the environment. The advanced oxidation processes are known as: Fenton, Fenton — photo, peroxon, catazon, Fenton- electrochemical,…The OH radicals are usually produced by activating hydrogen peroxide or ozone with various activating agents such as: transition metal ions, temperature, UV radiation,… [2], [4], [10], [12], [13], [19], [26]. Recent scientific announcements by scientists on the researching and application of other oxidants, such as persulfate and peroxymonopersulfate are also suitable for wastewater treatment containing persistent organic pollutant [8], [14], [15], [17]. If these oxidants are activated, they also produce free radicals which are higher oxidation activity than the original ones. Persulfates, peroxymonopersulfate are not stronger than hydrogen peroxide and ozone, but they are more durable than hydrogen peroxide and ozone in solution, better soluble in water than ozone. Specially, the process of activating the persulfate produces free radicals SO 4 and free radicals OH [8], [18], [25].
Azo dyes occupy more than 50 % of the dye global trade. Some azo dyes have been found to cause cancer, mutations in genes and are banned worldwide. However, they are still produced and used on a large scale in the dyeing industry now. Because they are low production cost, easy to synthesize and some good color properties. The bonds in the azo molecules are quite stable, showing the ability to decompose and accumulate in the environment [16], [19], [24].
The textile industry consumes a large amount of clean water and also discharges a similar amount of wastewater which is complex composition and properties. This wastewater contains residual dyes from dyeing process (occupying about 10 to 15 % of the dye initial amount) and has color, temperature, content of COD, BOD and surfactants being very high [7], [16], [19], [24]. Therefore, the treatment of azo dye wastewater is necessary and need to investigate. Some azo dyes were used in these experiments those are methyl orange.
Materials and methods
Chemicals
– Methyl orange (MO), purity of 99 %, Merck, Germany;
– Nano — zero valent iron powder (nZVI), purity of 99 %, d< 80 m, Fisher, Belgium;
– Sodium persulfate Na 2 S 2 O 8 (PS), purity of 99 %, Across, Belgium;
– Sodium hydroxide NaOH, purity of 99 %, Merck, Germany;
– Sulfuric acid H 2 SO 4 , purity of 99 %, Merck, Germany;
– Acetonitrile, ethanol, methanol with cleanliness for HPLC analysis, Fisher, Belgium;
– Twice distilled water.
Instruments
– High performance liquid chromatography (HPLC), Agilent 1100 Model G1314A Variable Wavelength UV-VIS Detector;
– pH-OAKLON machine, accuracy of 0.01, serie 510, USA;
– CHYO analytical balance, accuracy of 0.1mg, Japan;
– SB-348A air compressor, 1.5 L/min, China;
– UV light, capacity 15W, intensity of 875 Lux, wavelength 254 nm, USA;
– HY-5 shaking machine, shaking amplitude of 20mm, speed of 60 rpm, China;
– The system of self-made device of UV activated persulfate for oxidizing the AZOs: MO, AY, BT and waste water of textile dyeing villages.
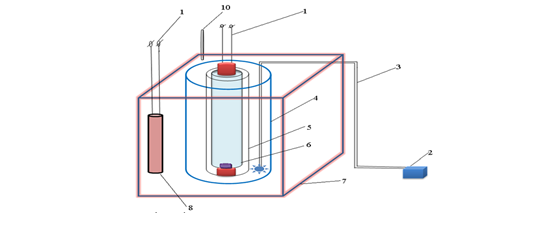
Fig. 1. Diagram of a reaction device for UV heated activated persulfate process
- Electricity supply 220V
- Air compressor
- Air duct
- Glass tube
- Quartz tube
- UV light
- Thermal bath
- Heat bar
- Thermometer
+ Operation principle:
Survey solution containing MO dye, nZVI powder and persulfate solution were added to the glass tube (4). The quartz tube (5) is put in the glass tube (4), inside the quartz tube is a UV lamp (6). The solution is stirred by an air compressor (2) through an air duct (3). The device works when the air compressor and the UV lamp are on.
In the event that heating is required to change the temperature of the reaction system, the UV activated persulfate device is placed in a thermostatic bath with water (7), with a heating bar (8) and the temperature adjusted and monitored (Fig. 1).
In the case that heating is required to change the temperature of the reaction system, the photo catalytic device is placed in a thermostatic bath with water (7), with a heating bar (8) and the temperature adjusted and monitored (Fig. 1).
Analytical methods
The concentration of MO in solution is determined by the HPLC. Retention times (t R ) of MO 2.6 minutes. The pH solutions were adjusted to be at pH= 4.5, temperature t= 25 C. Samples of survey solutions were collected in test tubes with a volume of 4 mL in test tubes always containing 1 mL of 0.01 M Na 2 S 2 O 3 solution, capping and shake well. After that, they are taken to determine the concentration of azo dyes with corresponding standards on HPLC machine (Agilent 1100 Model G1314A Variable Wavelength UV-VIS Detector)
The reaction rate of AZOs decomposition is calculated and based on the change of AZOs concentration over time, the formula:
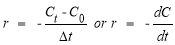
In which: r: Reaction rate;
C t , C 0 : Concentration of MO at time t and initial time;
t: Time variation.
Assuming the MO decomposition kinetics follows the pseudo first-order kinetics, reaction rate r, with the apparent reaction rate constant k p , the MO decomposition reaction rate is calculated according to the formula:
r = k p .C(1.2)
From formula (2.4) and (2.5) having:
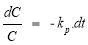
or
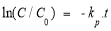
To study the kinetics of the decomposition reaction of AZOs according to equation (2.7). It is need to graph the dependency ln(C/C 0 ) — t. The slope of the line (1.74 is the apparent rate constant k p of the MO decomposition reaction in units (minutes -1 ).
Results and discussions
The kinetic of the MO decomposition in systems without UV and with UV
These are the nZVI/PS systems and ZVI/PS/UV systems to study the decomposition kinetic in Fig 2,3.
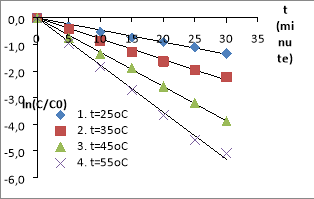
Fig. 2. The MO decomposition kinetics equations in the nZVI/PS/MO systems (The conditions: C ZVI = 0.5 g/L, C PS = 1.0 mM, C MO = 0.1 mM, pH= 4.5)
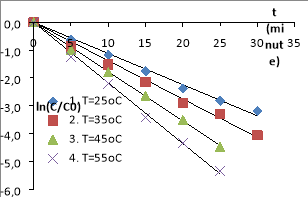
Fig. 3. The MO decomposition kinetics equations in the nZVI/PS/MO/UV systems (The conditions: C ZVI = 0.5 g/L, C PS = 1.0 mM, C MO = 0.1 mM, pH= 4.5, I= 785 Lux, = 254 nm)
The experiments show that the decomposition efficiency of MO in nZVI/PS/UV systems and nZVI/PS are quite high. The nZVI and the UV are activating PS to generate free radicals SO 4 −• , • OH. These free radicals have strong oxidation activity, have the effect of decomposing AZOs at high rate compared to normal oxidizing compounds, suitable for studies. The MO decomposition effect in the system with UV is higher than one without UV. Because the systems could happen reactions to form free radicals SO 4 −• , • OH [5], [20], [22], [24], [26].
Fe 0 + S 2 O 8 2- 2SO 4 2- + Fe 2+ , k= 1.5.10 M -1 .s -1
Fe 2+ + S 2 O 8 2- Fe 3+ + SO 4 + SO 4 2 , k= 2.0.10 1 M -1 s -1
Fe 2+ + SO 4 Fe 3+ + SO 4 2 , k= 3.0.10 8 M -1 s -1
SO 4 + SO 4 S 2 O 8 2- , k= 5.5.10 8 M -1 s -
SO 4 + H 2 O HSO 4 - + HO , k = 2.10– 3 M -1 s -1
SO 4 + HO HSO 4 - + 1/2O 2 , k= 1.5.10 7 M -1 s -1
SO 4 + S 2 O 8 2- S 2 O 8 - + SO 4 2- , k= 6.1.10 5 M -1 s -1
HO + HO H 2 O 2 , k= 5.5.10 9 M -1 s -1 ,
HO + S 2 O 8 2- S 2 O 8 - + OH , k= 1.2.10 7 M -1 s -1
HO + Fe 2+ HO + Fe 3+ , k= 4.3.10 8 M -2 s -1
Fe 2+ + H 2 O 2 Fe 3+ + HO + OH , k= 63.0 M -2 s -1
S 2 O 8 2- + UV 2SO 4 , k = 5.7.10– 5 s -1
References :
1 A. Kayode Coker (2010). Modeling of Chemical Kinetics and Reactor Design. Gulf Publishing Company, Texas, Printed in the United States of America.
2 Antoine Ghauch, Ghada Ayoub, Sahar Naim (2013). Degradation of pefamethoxazole by persulfate assisted micrometric Fe 0 in aqueous solution. Chemical Engineering Journal, Vol. 5, pp. 195–221.
3 Ana Maria Ocampo (2009). Persulfate activation by organic compounds. Doctor of Philosophy, Washington State University.
4 Bo-Tao Zhang, Yang Zhang, Yanguo Teng & Maohong Fan (2015). Sulfate Radical and Its Application in Decontamination Technologies. Environmental Science and Technology, Vol. 45, No. 16, pp. 1756–1800.
5 Chen-Ju Liang* and Shun-Chin Huang (2012). Kinetic model for sulfate/hydroxyl radical oxidation of methylene blue in a thermally-activated persulfate system at various pH and temperatures. Environmental Research, Vol. 22, no. 4, pp. 199–208.
6 Dawit Negash Wordofa (2014). Application of Iron Activated Persulfate for Disinfection in Water Treatment. Master of Science, University of California.
7 Faisal Ibney Haia, Kazuo Yamamotob and Kensuke Fukushi (2007). Hybrid Treatment Systems for Dye Wastewater. Environmental Science and Technology, Vol. 37, No. 4, pp. 315–377.
8 Guyu Shi (2015). Oxidation of 2,4-D using iron activated persulfate and peroxymonosulfate. Doctor of Science, Lowa State University.
9 Hannes Jónsson (2006). An introduction to Transition State Theory, Leiden University, Netherlands.
10 Holger Lutze (2013). Sulfate radical based oxidation in water treatment. Universität Duisburg-Essen.
11 Holger V.Lutze, Nils Kerlin, Torsten C. Schmidt (2015). Sulfate radical- base water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radical into hydroxyl radical and influence of bicarbonate. Water Research, Vol. 72, pp. 349–369.
12 Huanxuan Li, Jinquan, Wan (2013). Degradation of acid orange 7 by sulfate radicals generated from ZVI activated persulfate. Chemical Engineering Journal, Vol. 15, pp. 85–110.
13 J. M. Monteagudo., A. Duran, R. Gonzalez, A. J. Exposito (2015). In situ chemical oxidation of carbamazepine solutions using persulfate simultaneously activated by heat energy, UV light, Fe 2+ ions, and H 2 O 2 . Applied Catalysis B: Environmental, Vol. 176, pp. 120–129.
14 Jiabin Chen, Liming Zhang, Tianyin Huang∗, Wenwei Lib, Ying Wanga,Zhongming Wang (2016). Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: Radical versus non-radical mechanism. Journal of Hazardous Materials, Vol. 571–580, pp. 320.
15 Kanwartej S. Sra, Jessica J. Whitney, Neil R. Thomson, and Jim F. Barke (2010). Persulfate Decomposition Kinetics In The Presence. University of Waterloo, Canada.
16 KlausHunger (2002). Industrial Dyes Chemisry, Properties, Applications. Wiley-VCH publisher, Frankfurt, Germany.
17 Li Zhao, Yuefei Ji, Deyang Kong, Junhe Lua, Quansuo Zhoua (2016). Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process. Chemical Engineering Journal, Vol. 303, pp. 458–466.
18 M. Peluffo, F. Pardo, A. Santos a, A. Romero (2016). Use of different kinds of persulfate activation with iron for the remediation of a PAH-contaminated soil. Science of the Total Environment, Vol. 563, pp. 649–656.
19 Masoumeh Beikmohammadi, Mehdi Ghayebzadeh, Kiomars Shrafi, and Esmaeil Azizi (2016). Decolorization of Yellow-28 Azo dye by UV/H 2 O 2 advanced oxidation process from aqueous solutions and kinetic study. International Journal of Current Science, Vol. 19, pp. 126–132.
20 Minghua Nie, Caixia Yan, Meng Li, Xiaoning Wang, Wenlong Bi, Wenbo Dong (2015). Degradation of chloramphenicol by persulfate activated by Fe2+ and zero valent iron. Chemical Engineering Journal, Vol. 279, pp. 507–515.
21 Minghua Nie, Caixia, Mengli (2010). Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Environmental Engineering Journal, Vol. 25, pp. 55–76.
22 Parmila Devi, Umashankar Das, Ajay K. Dalai (2016). In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Science of the Total Environment, Vol. 571, pp. 643–657.
23 Paul Tratnyek, Jamie Powell, Rachel Waldemer (2009). Improved Understanding of In Situ Chemical Oxidation Contaminant Oxidation Kinetics, Oregon Health & Science University, USA.
24 Xiang-Rong Xu, Xiang-Zhong Li (2010). Degradation of azo dye orange G in aqueous solutions by persulfate with ferrous ion. Separation and purification technology, Vol. 72, No. 1, pp. 105–111.
25 Yi Yang, Jin Jiang,* Xinglin Lu, Jun Ma,* and Yongze Liu (2015). Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process. Environment Science Technology, Vol. 49, p. 7330−7339.
26 Yiqing Zhang, Jiefeng Zhang, Yongjun Xiao, Victor W. C. Chang, Teik-Thye Lim (2016). Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H 2 O 2 and UV/persulfate. Chemical Engineering Journal, Vol. 302, pp. 526–534.







