}}}
The goal of the study was to ascertain the optimal conditions for Monascus purpureus to produce pigments through biosynthesis. Peptone, glucose, a C/N ratio of 8, pH values of 4 for yellow pigment and pH values of 5.5 for red pigment were found to be the ideal medium for Monascus purpureus to create both yellow and red pigments. The yield of red and yellow pigments was 1271.12±96.58 AU/g and 3996.30±2.41 AU/g, respectively. The M. purpureus pigment extract showed antibacterial activity against E. coli and significant antioxidant activity with an efficiency of 87.86 ± 0.40 %.
Keywords: Monascus purpureus, culture medium, pigment, nitrogen, carbon, pH
Pigments play an essential role in the health of humans and animals thanks to their ability to enhance the immune response, capture oxidative radicals, and protect cells. Natural pigment is more acceptable to consumers than synthetic pigment due to its safety. Diversification of natural pigment sources will support the use of safe pigments for health and improve the nutrient content of products. Production of natural pigments such as carotenoids, anthocyanins, and chlorophyll can be achieved through extractions from plants and animals or by synthesizing fermentation of yeast and algae (Mapari et al., 2009; Dufossé et al., 2014).
The Monascus mold genus can produce yellow and red pigments that can be used to color food. Monascus has have been widely used in food production in China, Korea, and Japan for hundreds of years (Wang et al., 1997). The pigments of Monascus purpureus are mixtures of red, orange, and yellow compounds, classified into polyketides. Monascin and Ankaflavin — yellow pigment (Chen et al., 1969; Manchand and Whalley, 1973); Rubropuncatin and Monascorubrin — orange pigment (Chen et al., 1969; Manchand & Whalley, 1973); Rubropuncamine and Monascorubramine — red pigment (Kumasaki et al., 1962; Sweeny et al., 1981) are six well-known Monascus purpureus pigments. Monascus fungi synthesize pigments through the polyketide biosynthesis pathway, in which polyketide synthase and fatty acid synthase play essential roles (Jůzlová et al., 1996). Of the various Monascus pigments, the yellow and red ones are particularly significant because red is often the preferred food coloring.
In Vietnam, research on the pigment-creating ability of Monascus purpureus microfungi is limited. With the desire to develop a native Monascus purpureus fermentation process capable of biosynthesizing high pigment content, we carried out research investigating the influence of several factors on the ability of Monascus purpureus fungi to produce red and yellow pigments.
2. Subjects and methods of research
2.1. Subjects of research
Monascus purpureus is provided by the Microbiology Technology Laboratory, Department of Biology — Environment, University of Science and Education, The University of Da Nang.
2. 2. Research methodology
Culture method: Cut a circle of Monascus purpureus mushroom about 1cm into 100 ml of liquid PDA medium, dark culture at 25°C, shake 125 rpm, and keep the environmental pH at 6.5 for seven days.
Investigated the influence of several factors on the pigmentation capacity of the fungus Monascus purpureus :
— Nitrogen and carbon sources: Mold strains were cultured for seven days on PDA media, collecting biomass and determining the pigment content produced.
+ Nitrogen source: With the addition of different nitrogen sources (peptone, NH 4 NO 3 , NaNO 3 , (NH 4 ) 2 SO 4 ) with a concentration of 0.5 % (w/v), the control sample is a nitrogen-free PDA medium
+ Carbon source: added lactose, sucrose, maltose, fructose instead of glucose with a concentration of 2 % (v/v)
— C/N ratio: Select the appropriate nitrogen vac carbon source from the above experiment to investigate. Change the C/N ratio to 4, 6, 8, 10, and 12. Transplant and shake them for seven days and then collect biomass to calculate the pigment content produced
— Effect of pH: change the pH value of the culture medium (4, 4.5, 5, 5.5, and 6, respectively) after 7 days of biomass collection and determination of pigment content produced.
— Method of pigment extraction from fungi: The extracted pigment content was determined by Lin and Demain, 1991.
— Methods for investigating antioxidant and antimicrobial activity
+ The ability to eliminate free radicals is determined by the ABTS + decolorization method (Re et al., 1999). ABTS + solution consists of 7 mM ABTS solution and 10 mL 2.45 mM K 2 S 2 O 8 solution. The solution was incubated for 16 hours in a dark environment and then diluted with ethanol to obtain an optical density of 0.7±0.05 at a wavelength of 734 nm. ABTS + free radical neutralization activity was investigated by adding 10 μL of pigment extract of Monascus purpureus to 990μL of ABTS + . The reaction mixture was annealed for 6 minutes, and spectral absorption was measured at a wavelength of 734 nm.
The ABTS free radical capture capacity of carotenoid extracts is expressed through the inhibition rate (I %) and is calculated by the formula:
I % = [(Ao-As)/Ao] × 100 %,
where Ao: optical density value of white sample;
As: optical density value of test specimen
— Microbial strain: Escherichia coli (ATCC 25922) provided by the Faculty of Medicine and Pharmacy, University of Da Nang.
The pigment of Monascus purpureus is dissolved in DMSO. In the experiment, the controls used include: negative control is DMSO, positive control is tetracycline; suck 100 μL of test substance into the agar well, the petri dishes are incubated for 24 hours at 37 o C. After that, the diameter of the inhibition zone is evaluated. If there is an inhibition zone, it proves that the test substance has the ability to resist the tested bacterial strain (Ferdes et al., 2009).
— Data processing methods: The metrics are processed and determined by ANOVA and T-test statistical significance using SPPSS 22.0 data processing software, with a p-value.
3. Results and discussion
3.1. Investigation of the influence of nitrogen sources on the ability of Monascus purpureus fungi to produce red and yellow pigmentation
The results of the study on the influence of nitrogen sources (pepton, NH4NO3, NaNO3, (NH4)2SO4 of Monascus purpureus fungi to produce red and yellow pigments are shown in Figure 1.
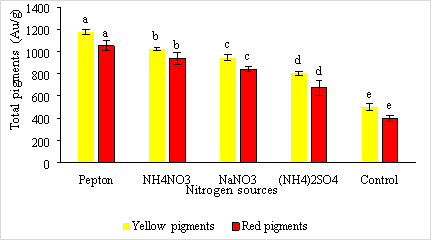
Fig. 1. The effect of nitrogen sources on the ability of fungi to produce red and yellow pigmentation of Monascus purpureus
Nitrogen sources have been shown to influence cell growth, quantity and quality, and microbial pigmentation. According to Chen (1696), using pepton as an organic protein source in the fermentation medium stimulates biomass and the amount of red pigment formed. According to Jun-Sung Kim et al. (1993), organic protein sources are more suitable for growth and pigment formation than inorganic protein.
Results from Figure 3.1. shows that most nitrogen sources are favorable for red and yellow pigmentation. In particular, pepton is the most suitable source of nitrogen for yellow and red pigmentation with the highest concentrations of 1174.03 ± 22.69e AU/g and 1052.56 ± 44.24e AU/g respectively, which has shown that nitrogen sources have a positive effect on the amount of pigment produced. These results are consistent with the findings of authors Chen and Johns (1994), Silviera et al. (2008).
According to Chen, Mh and Johns (1994) among nitrogen sources such as nitrates, ammonium salts, peptides and ammonium salts are better suited for pigment growth and synthesis than nitrate salts. In addition to (NH4) 2 SO 4 being a source of inorganic nitrogen, the amount of pigment obtained is very low with the amount of yellow and red pigments being 802.00 ± 17.23 b AU/g and 673.613 ± 69.05 b AU/g, respectively.
As such, pepton is the most suitable source of nitrogen for red and yellow pigmentation when added to the environment because it gives the highest amount of biomass.
3.2. Investigation of the effect of carbon sources on the ability of Monascus purpureus fungi to produce red and yellow pigmentation
Carbon hydrates have been used as carbon and energy sources for pigment formation in Monascus fungi . In this experiment, five main carbon sources, glucose, fructose, maltose, sucrose, and lactose are used to investigate the influence of carbon sources on pigment formation in this fungi. The results are obtained as shown in Figure 2.
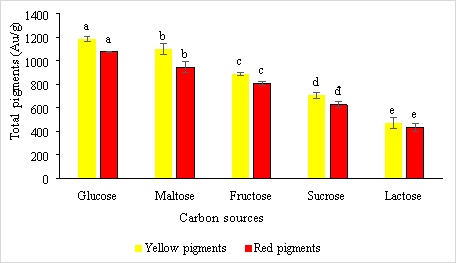
Fig. 2. The effect of carbon sources on the formation of red and yellow pigments by the fungus Monascus purpureus
Results from Figure 3.2 shows that glucose is a suitable carbon source for the formation of yellow and red pigments with concentrations of 1182.29 ± 20.86 e AU/g and 1076.72 ± 6.38 e AU/g, respectively. This result is consistent with research by Joshi et al., 2003, when they identified glucose and its oligosaccharides as the best source of carbon for both growth and pigment production minus fructose. Suraiya and colleagues (2018) optimized the response surface methodology and found that 1.32 % glucose addition was optimal for solid medium. But Chen and Johns (1994) report that high maltose levels enhance red pigment production three times more than using high glucose levels.
3.3. Investigation of the effect of the C/N ratio on the ability of Monascus purpureus fungi to produce red and yellow pigmentation
The C/N ratio also affects the synthesis of red and yellow pigments by the Monascus fungi. When selecting the most optimal source of nitrogen and carbon to survey the C/N ratio, the results are presented in Figure 3.
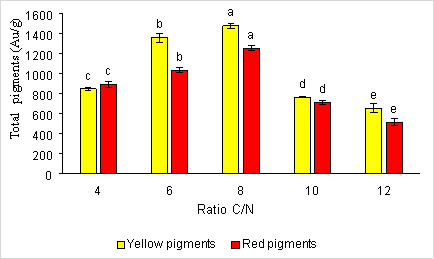
Fig. 3. The effect of the C/N ratio on the formation of red and yellow pigments by fungi Monascus purpureus
Result from figure 3.3 shows C/N ratio is equal to 8, therefore the pigment content formed is very high with yellow and red pigment content of 1481.04 ± 26.95 e AU/g and 1254.93 ± 23.99 e AU. Miyake and his colleagues also combined carbon sources and nitrogen sources in different ratios to ferment pigments. The results showed that combining carbon and nitrogen sources in different ratios, the optimal carbon-to-nitrogen (C/N) ratio is between 7–9. Meanwhile, at a ratio of C/N equal to 12, the amount of pigment produced is low with the amount of yellow pigment 656.933 ± 42.14 a AU/g and the amount of red pigment 514.027 ± 34.26 a AU/g.
3.4. Investigation of pH effects on the ability of Monascus purpureus fungi to produce red and yellow pigmentation
Environmental pH plays a vital role in activating major enzymes involved in red pigment production and citrinin secretion in Monascus purpureus CCT3802. The results of the survey are presented in Figure 4.
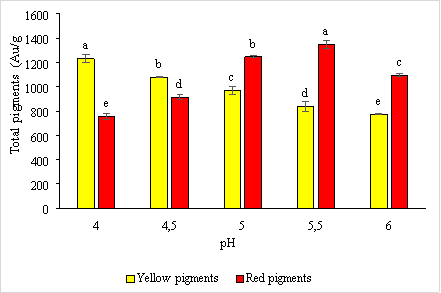
Fig. 4. The effect of pH on the synthesis of red and yellow pigments by the microfungus Monascus purpureus
Results from Figure 3.4 shows that at pH = 4 the highest amount of yellow pigment produced with 1231.27 ± 33.04 e AU/g, at pH = 5.5 the amount of red pigment produced with the highest content of 1346.43 ± 34.07 e AU/g. This result is consistent with research by Yongsmith et al. (1993) and Johns and Stuart (1991).
According to research by Yongsmith et al. (1993), at pH = 4, there is a stimulation of the synthesis of ankaflavin (yellow pigment), and the total amount of pigment formed gradually increases with increasing pH to 5.5. At pH = 6, the amount of yellow pigment produced is low, with a content of 772.813 ± 6.85 a AU/g; at pH = 4, the amount of red pigment produced is low, with a content of 755.213 ± 27.89 a .
3.5. Antioxidant activity of Monascus purpureus
The ABTS + free radical neutralizing effect is shown in table 1 and figure 5.
Table 1
Antioxidant activity of the fungus Monascus purpureus
|
Optical Density |
Efficiency % |
|
|
Control Sample (-) |
0,6222 ± 0,003 c |
11,11 % ± 0,413 a |
|
Pigment Extract Sample |
0,085 ± 0,003 b |
87,86 % ± 0,402 b |
|
Vitamin C Sample (+) |
0,0524 ± 0,001 a |
92,51 % ± 0,111 c |

Fig. 5. ABTS+ color change of pigment and vitamin C extraction samples
The results in Table 3.1 and Figure 3.5 show that the optical density of the Monascus purpureus extract sample was lower than that of the negative control sample, indicating that the fluid can capture free radicals. The optical density and free radical capture efficiency of Monascus purpureus extracted samples were 0.085±0.003 and 87.86 %±0.40 %. However, the antioxidant capacity of Monascus purpureus extract is 4.65 % lower than vitamin C (high antioxidant). Chen et al. (2021) investigated the antioxidant capacity of pigment extracts from fermented fish bones with Monascus purpureus for 3 and 6 days with free radical capture efficiencies of 92 % and 93 %, respectively. This result was already higher than the results of this study. This may be because the increase in the total phenolic and amino acid content during fermentation supported the antioxidant capacity of the pigment extracted from fish bones.
The antioxidant capacity of the red pigment and yellow pigment of Monascus purpureus strain may be related to monasfluore B, monascin, and rubropunctamine. These pigments are known to be potent antioxidant compounds (Dahle et al., 2007).
3.6. Antibacterial activity of Escherichia coli of the fungus Monascus purpureus
|
Sample |
Diameter of Inhibition Zone (mm) |
|
Pigment Extract |
7,50±0,96 a |
|
DMSO (-) |
0,00±0,00 |
|
Antibiotic (+) |
21,0±0,15 b |
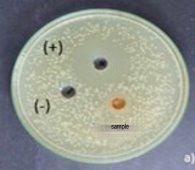
Fig. 6. Antibacterial activity of pigment extract of strain Monascus purpureus
The study results showed that the pigment extract of Monascus purpureus strain was resistant to E. coli . The diameter of the inhibition zone is 7.50±0.96 mm, respectively. According to Ferdes et al. (2009), the yellow pigment of the M. purpureus M5 strain extracted by n-hexane has the ability to inhibit B. subtilis B1, B. subtilis B2, P. aeruginosa P1, P. aeruginosa P2, E. coli with inhibition zone diameters of 11, 12, 9, 9, 9, 9 mm, respectively. Kaur et al. (2023), when studying the antimicrobial activity of the Monascus purpureus CPEF02 strain performed using a disc diffusion assay, showed inhibitory zones against four bacterial pathogens, namely, S. aureus,S. typhimurium , S. aureus resistant to methicillin and V. cholerae with inhibition zone diameters of 9.33 ± 0.33mm, 7.68 ± 0.2mm, 6.42 ± 0.15mm, 6.9 ± 0.33mm, respectively.
4. Conclusion
Research has determined the optimal environment for Monascus purpureus fungi to produce yellow and red pigments: nitrogen source is pepton, carbon source is glucose, ratio C/N = 8 at pH = 4 (for yellow pigment); pH = 5.5 (for red pigment) with red pigment and yellow pigment content achieved at 1271.12±96.58 AU/g and 3996.30±2.41 respectively AU/g. The pigment extract of the Monascus purpureus strain has potent antioxidant activity with efficiencies of 87.86 ± 0.40 % and is simultaneously resistant to E. coli bacteria.
Chen, F. C., Manchard, P. S., & Whalley, W. B. (1969). The structure of monascin. Journal of the Chemical Society D: Chemical Communications , (3), 130–131.
References:
- Chen, Y. T., Hsieh, S. L., Gao, W. S., Yin, L. J., Dong, C. D., Chen, C. W., Singhania, R. R., Hsieh, S., & Chen, S. J. (2021) Evaluation of Chemical Compositions, Antioxidant Capacity and Intracellular Antioxidant Action in Fish Bone Fermented with Monascus purpureus . Molecules , 26 (17), 5288.
- Dahle, M. A., Divakar, S., Kumar, S. U., & Vijayalakshmi, G. (2007) Isolation and Characterization of Dihydromonacolin-MV from Monascus purpureus for Antioxidant Properties. Appl Microbiol Biotechnol.,73 (5), 1197–1202.
- Dufossé, L., Fouillaud, M., Caro, Y., Mapari, S. A., & Sutthiwong, N. (2014). Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Current Opinion in Biotechnology , 26 , 56–61.
- Ferdes, M., Ungureanu, C., Radu, N., & Chirvase, A. (2009). Antimicrobial effect of Monascus spp. red rice against some bacterial and fungal strains. Chemical Engineering Transaction , 17 , 1149–1154.
- Jůzlová, P., Martinkova, L., & Křen, V. (1996). Secondary metabolites of the fungus Monascus: a review. Journal of industrial microbiology and biotechnology , 16 (3), 163–170.
- Kaur, M., Goel, M., Mishra, R. C., Lahane, V., Yadav, A. K., & Barrow, C. J. (2023) Characterization of the Red Biochromes Produced by the Endophytic Fungus Monascus purpureus CPEF02 with Antimicrobial and Antioxidant Activities. Fermentation , 9 (4), 328.
- Kim, J. S., Choi, K. H., Choi, J. Y., Lee, Y. S., & Chang, Y. Y. (1993). Induction of a Mutant, Monascus anka 732Y3 from Monascus anka KFCC 11832 and its Morphological Observations. Journal of Microbiology and Biotechnology , 3 (2), 134–138.
- Kumasaki, S., Nakanishi, K., Nishikawa, E., & Ohashi, M. (1962). Structure of monascorubrin. Tetrahedron , 18 (10), 1171–1184.
- Lin, T. F., & Demain, A. L. (1991). Effect of nutrition of Monascus sp. on formation of red pigments. Applied Microbiology and Biotechnology , 36 (1), 70–75.
- Manchand PS, Whalley WB (1973). Isolation and structure of ankaflavin: a new pigment from Monascus anka. Phytochemistry , 12 , 2531–2532.
- Mapari, S. A., Meyer, A. S., Thrane, U., & Frisvad, J. C. (2009). Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microbial Cell Factories, 8(24). DOI: 10.1186/1475–2859–8-24.
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med , 26 (9–10), 1231–1237.
- Sweeny, J. G., Estrada-Valdes, M. C., Iacobucci, G. A., Sato, H., & Sakamura, S. (1981). Photoprotection of the red pigments of Monascus anka in aqueous media by 1, 4, 6-trihydroxynaphthalene. Journal of agricultural and food chemistry , 29 (6), 1189–1193.
- Yongsmith, B., Tabloka, W., Yongmanitchai, W. and Bavavoda, R. (1993), “Culture conditions for yellow pigment formation by Monascus sp. KB 10 grown on cassava medium”, World Journal of Microbiology and Biotechnology, 9 , pp. 85–90.
- Wang, J., Lu, Z., Chi, J., Wang, W., Su, M., Kou, W.,... & Chang, J. (1997). Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (red yeast) rice preparation from traditional Chinese medicine. Current Therapeutic Research , 58 (12), 964–978.







