The average individual effective doses, received by six million residents of the contaminated areas after the Chernobyl accident during the whole period 1986–2005 were around 9 mSv, which means that “most of the workers and members of the public were exposed to low level radiation comparable to, or at most a few times higher than, the annual natural background levels.” [1]. It was estimated that individual external and internal effective doses received by the residents of Kiev during the first year after the Chernobyl accident were about 3 mSv and 1.1 mSv respectively, decreasing in the following years [2], thus being comparable with the global average annual doses from the natural radiation background (2.4. mSv). Nevertheless, patients from Kiev were repeatedly studied together with residents of contaminated areas within the 'exposed' cohorts [3,4]. The worldwide annual exposures to the natural background radiation vary widely; they are generally expected to be in the range 1–10 mSv [5] but are higher in some densely populated areas [1,6,7]. High natural radiation background is not known to be associated with any increase in health risks [5,8,9], leaving apart the separate topic of radon and lung cancer at a cumulative exposure level of about 250 mSv [10]. The average individual doses from the background radiation for some countries are given e. g. in [11]. This matter should have been elucidated in the publications where patients from different countries were compared; otherwise exposures in a control group can turn out to be not significantly different from those in the exposed cohort, as it was the case for the patients from Kiev vs. those from Spain [3,4]. A comparison with controls from West Europe should also have included dose estimates from diagnostic radiology extensively used in the West. So, a computed tomographic (CT) examination causes an effective dose 2–20 mSv, while the doses from interventional CT procedures usually range within 5–70 mSv [12].
In the study [13], the patients were subdivided according to the soil contamination: 1st group — 5–30 Ci/km2 (185–1110 kBq/m2); 2nd group — 0.5–5 Ci/km2 (18.5–185 kBq/m2). Individual whole body lifetime doses as a function of the soil contamination were estimated as follows: for the range 185–555 kBq/m2–5–20 mSv; for 555–1480 kBq/m2–20–50 mSv [11]. Dose estimates for different soil types, compatible with or somewhat higher than the above figures (with no account taken of current countermeasures), are given in [14]: for the period 1986–2000 the dose range was from 2 mSv in towns located in black soil areas with the contamination level 40–600 kBq/m2 up to 300 mSv in villages with podzol sandy soil with contamination 600–4000 kBq/m2. The doses expected for the period 2001–2056 are considerably lower. For comparison, the standard (70 years) lifetime dose from the average natural radiation background (2.4 mSv/year) is 170 mSv, with a typical range 70–700 mSv for different regions [14]. The above comparisons show that the term 'chronic, long-term, low doses of ionizing radiation', used e. g. in [3,4,13,15–17] is not generally applicable to the residents of contaminated areas after the Chernobyl accident. However, individual doses to the thyroid were higher than the whole body doses approximately by a factor of ten [11), while the average thyroid dose to pre-school children was 2-4 times higher than the population average [1].
A statement in [4]: 'Recent studies have shown that during the period subsequent to the nuclear Chernobyl accident (April 1986), an increase in morbidity (4.7 to 9.8 per 100,000 of the total population), aggressiveness, and proliferative activity of renal cell carcinomas (RCCs) from Ukrainian patients is recognized' was endorsed by a self-reference to [15] and another reference to a report by the Ukrainian Ministry of Health. However, no cancer incidence increase, apart from thyroid carcinoma (TC) among people exposed during their childhood or adolescence, has been proven to result from the Chernobyl exposures [1,18]; while among the causes of the registered TC increase were improved medical surveillance, reporting, and regular examinations [1]. The increase in 'aggressiveness' was obviously caused by detection with the help of improved diagnostics and the screening of old neglected cancers, sometimes misinterpreted as radiogenic tumors developing after a short latency. Besides, some cases could have been brought from non-contaminated areas, because it was generally known that diagnostics in the contaminated areas had been improved. Morphologic and molecular-genetic differences between renal cancers from contaminated and non-contaminated areas were probably caused by differences in tumor grade and stage between the compared cohorts: cancers from Ukraine tended to be more advanced and accordingly less differentiated than the controls from Spain [3,4,15]. In its turn, it was obviously caused by earlier detection of malignancies in Spain.
High aggressiveness, invasiveness or poor differentiation of TC in patients from contaminated areas was reported by many studies, some of them referenced in [19]; while the authors of the latter work found no enhanced aggressiveness of TC in a large cohort of patients with TC developed after radiotherapy for benign conditions. It is in agreement with the concept, according to which a later detection has contributed to the 'aggressive' features of Chernobyl-related cancer. The following was stated in a recent review: 'Despite early reports suggesting that the pediatric thyroid cancer cases that developed after exposure to Chernobyl fallout were particularly aggressive, it now seems that the initial presentation and early clinical course of most of these cases are very similar to both non-radiation-associated pediatric thyroid cancers and thyroid cancers that arise after exposure to external beam irradiation' [20]. At the same time, 'at diagnosis, 60–70 % of the Chernobyl-related pediatric thyroid cancers had clinically evident cervical lymph node metastases (N1)' [20]. This percentage is comparable with some reported data on the metastasizing of pediatric TC [21] and higher than by other researchers [22,23], being obviously high enough to be compatible with the concept of aggressiveness of post-Chernobyl TC [24,25], or, alternatively, of a relatively advanced stage of these tumors at diagnosis. Furthermore, the following statement can be misleading: 'With regard to the size of the primary tumor, 77 % were greater than 1 cm, suggesting that these were not incidental thyroid cancers detected by aggressive screening.' [20]. In fact, mass screening detected not only small incidental cancers but also advanced TC, not detected before because of the incomplete coverage of the population by medical checkups; more details are in [26–32]. This predictable phenomenon was confirmed by the fact that the 'first wave' TC after the Chernobyl accident were on average larger and significantly less structurally differentiated than those detected later [25].
Another misunderstanding can arise from the article [33], containing the following phrase in the open access abstract: 'Apart from the dramatic increase in TC incidence among those exposed at a young age and some increase of leukemia and solid cancer in most exposed workers, there is no clearly demonstrated increase in the somatic diseases due to radiation.' However, in the Chernobyl Forum publication [14] quoted in [33], leukemia and solid cancers (other than TC) are not discussed. In another Chernobyl Forum publication [34] it is stated that 'apart from the dramatic increase in TC incidence among those exposed at a young age, there is no clearly demonstrated increase in the incidence of solid cancers or leukemia due to radiation in the most affected populations' and further 'there have been many post-Chernobyl studies of leukemia and cancer morbidity in the populations of contaminated areas in the three countries. Most studies, however, had methodological limitations and lacked statistical power. There is therefore no convincing evidence at present that the incidence of leukemia or cancer (other than thyroid) has increased in children, those exposed in utero, or adult residents of the contaminated areas.' [34]. Admittedly, evaluation of the health effects was not the main goal of the IAEA Chernobyl Forum publications [14,34]. In the Report of the UN Chernobyl Forum Expert Group 'Health', it was commented that 'there is currently no evidence to evaluate whether a measurable risk of leukemia exists among the exposed as adults in the general population... With regard to the liquidators, there is clearly a need to clarify the existing observations' and further 'there is no evidence of increased risk of non-thyroid solid cancers resulting from Chernobyl' [35]. The same, in principle, is said in the text of the article [33]. So, the above-cited statement from the open access abstract is substantiated neither in the article text nor in the Chernobyl Forum publications directly or indirectly referred to in this article named 'The Chernobyl Forum: major findings and recommendations' [33], in fact amounting to misquoting. Furthermore, the counterpart of the 'the most exposed workers' or liquidators [33] in the general population, middle-aged men from the working class, are incompletely covered by medical services, so that regular medical checkups of the exposed workers have predictably resulted in the increase of the registered incidence of different diseases. These considerations, as well as the probable bias due to the dose-dependent self-reporting of the patients knowing their dose estimates [36], pertain also to the recent study [37], where national statistics for leukemia in men were used as an external control for a cohort of liquidators.
TC was relatively rarely diagnosed in children and adolescents in the former SU before the Chernobyl accident: in Belarus during 1981–85 the absolute number of TC diagnosed in children under 15 years was 3, and the corresponding annual rate per million children under 15 years — 0.3; for Ukraine correspondingly 25 and 0.5 [38]. For the northern regions of Ukraine contaminated after the Chernobyl accident these values were correspondingly 1.0 and 0.1 [38]. The same values are given in the IARC publication [39]. The above incidence rates are relatively low in comparison with those for other developed countries. TC is the most frequent tumor of endocrine glands in children and adolescents; its incidence was estimated to be 2–5 per 1 million per year [40]. Based on the cases diagnosed during the period 2000–2004, the US Cancer Registry SEER reported an annual age-adjusted incidence rate 8.5 per 100,000, approximately 2.1 % of the cases being diagnosed under the age 20 [40]; which corresponds to the annual incidence rate in the latter age group around 1.8 per 1 million. Corresponding data from a regional Tumor Registry in Würzburg, Germany, are given in the same article, where age-adjusted incidence rate per 1 million for the age under 20 years was 2.0 [40]. Illustrative are the numerical data from [41], available on-line as a table [http://www.scielo.br/img/revistas/abem/v51n5/a11tab1f.gif] [42], comparing pediatric TC incidence in different countries. An extract is presented here as the Table I.
Table I
Thyroid cancer incidence in children 0–14 years old [38, 41, 42]
|
Country (region) |
Period |
Incidence rate per million |
Age standardized incidence rate |
|
Belarus |
1981–85 |
0.3 |
- |
|
Ukraine |
1981–85 |
0.5 |
- |
|
Ukraine (North) |
1981–85 |
0.1 |
- |
|
Bulgaria |
1980–89 |
0.7 |
0.6 |
|
Canada |
1982–91 |
1.6 |
1.4 |
|
Costa Rica |
1984–92 |
0.7 |
0.7 |
|
Czech Republic |
1980–89 |
1.1 |
0.9 |
|
Nigeria (Ibadan) |
1983–92 |
0.2 |
0.2 |
|
Norway |
1980–89 |
1.4 |
1.2 |
|
Thailand |
1983–93 |
0.8 |
0.7 |
It can be seen from the Table 1 that incidence of pediatric TC is higher in more developed countries, obviously in consequence of the better diagnostics. Comparing these figures with the above for Belarus and Ukraine, it is evident that there must have been a pool of undiagnosed TC in the former SU before the Chernobyl accident. In Russian Federation, TC was started to be registered as a separate entity only in 1989 [43], when the screening after the Chernobyl accident had been initiated and the registered TC incidence began to increase. Percentage of older, more advanced cancers must have been higher among the 'first wave' cases after the accident, when the pool of neglected cancers had been untapped, equipment of histopathological laboratories not modernized, and post-Chernobyl «oncological alertness» was at its apogee.
Furthermore, the chromosomal rearrangement of the tyrosine kinase proto-oncogene RET/PTC3 was found to be more frequent in TC of non-exposed patients from Ukraine than in TC from France: 64.7 % vs. 42.9 % [44], which was probably caused by earlier tumor detection in France. Remarkable data were reported about thyroid adenoma: the RET rearrangements were found in 57.1 % of the non-exposed patients from Ukraine and in absolutely 0 % in adenomas from France. Such a difference between the specimens from Ukraine and France can be explained by a remark from the same article that at a re-examination, in some of the adenomas from Ukraine (but not from France) were found groups of cells with nuclear features of papillary thyroid carcinoma [44], which sounds unusual and indicates uncertainty of the histopathological diagnostics. This is a possible explanation for another paradox: in different groups of men with benign prostatic hyperplasia (BPH) and women with chronic cystitis, from contaminated areas and Kiev, severe urothelial dysplasia and carcinoma in situ (CIS) were found by a bladder biopsy as frequently as in 56–92 % of all random cases [13,16,17]; while the random selection mode was pointed out: «The Institute of Urology (Academy of Medical Sciences of Ukraine) in Kiev during 1994–2006 collected all benign prostate hypertrophy (BPH) patients who underwent suprapubic prostatectomy, and all these patients were included in our study in different years without exception» [13]. Furthermore, the following was stated about patients with BPH studied by bladder biopsy: 'Irradiation cystitis with multiple foci of severe urothelial dysplasia/CIS and some invasive transitional cell carcinoma were observed in 96/66, 76/56 and 56/8 % of patients in groups I, II and III respectively.' [45]. In the Handout by the same authors, distributed at the XXIII International Congress of the International Academy of Pathology (IAP) on 15–20 October 2000 in Nagoya, Japan, the following was written: 'Histologically the different forms of proliferative cystitis, which were frequently combined and had features of irradiation cystitis with multiple areas of severe dysplasia and carcinoma in situ (CIS), sometimes associated with small transtional-cell carcinoma (TCC), occurred in 97 % of patients from the radiocontaminated areas of Ukraine.' Such a high prevalence of CIS in randomly selected BHP patients is obviously unrealistic and is indicative of false-positivity. Note that overdiagnosis of dysplastic and neoplastic lesions can entail overmanipulation and overtreatment [31]. The overdiagnosis could have taken place also earlier, which appears probable looking at an illustration e. g. in [46]. Further discussion is in the papers [29–31], which have never been cited by the authors [13,15–17]. In the studies of the bladder lesions [13,15,17] there were no comparisons with controls from other countries; while the differences between the exposed and non-exposed groups could have been caused by a selection mode and quality of specimens. In this regard, the illustrations from the article [13] available online under [http://carcin.oxfordjournals.org/content/30/11/1821.long], should be commented. These images are reproduced here to prevent false-positivity in future (Fig. 1,2).
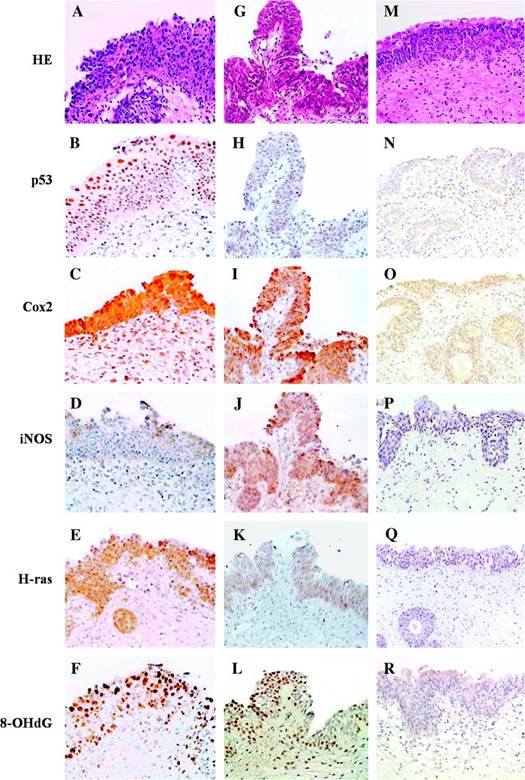
Fig. 1. From the caption: 'Small developing papillary urothelial carcinoma with severe dysplasia (G-L)'. [13] The nuclei are insufficiently stained. Neither severe dysplasia nor carcinoma are recognizable. A small papilloma cannot be excluded.
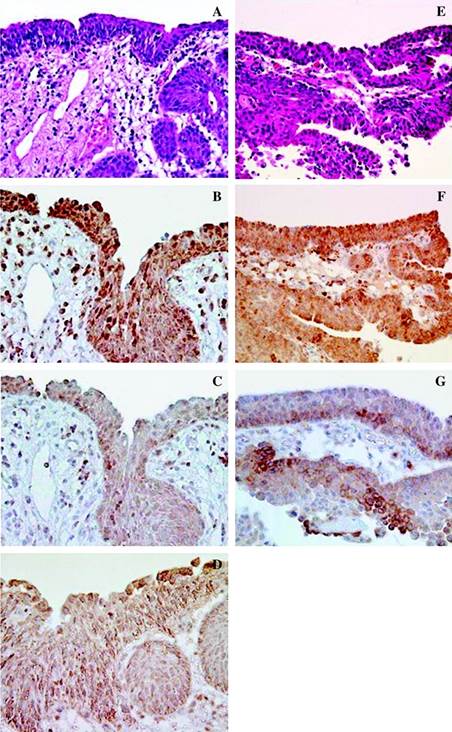
Fig. 2. From the caption: 'Imunohistochemical findings for a group 1 male with dysplasia (A-D) and small papillary urothelial carcinoma (E-G).' [13] Thick sections, some nuclei are weakly stained. Severe dysplasia and carcinoma are not recognizable.
All the slides (Fig. 1,2) are obviously too thick for reliable diagnostics. Insufficient quality of specimens could have been caused also by fixation and processing-related factors, thermal tissue damage due to electrocoagulation etc. Finally, radiation-induced changes of squamous and transitional epithelium, known from studies of biopsies from patients after therapeutic irradiation, are absent in these and other images, which is commented in the article: 'Classic descriptions of acute and chronic radiation effects on the urinary bladder do not coincide with the pathogenesis of human urinary bladder injury after long-term, low-dose exposure to IR' [13]. It is an additional argument against ionizing radiation as a causative factor. Histological images of the bladder mucosa and thyroid, potentially conductive to false-positive conclusions about malignancy, can be seen in the widely used editions [46,47] on tumor histopathology (Fig. 3,4); further similar images were reproduced in [48].
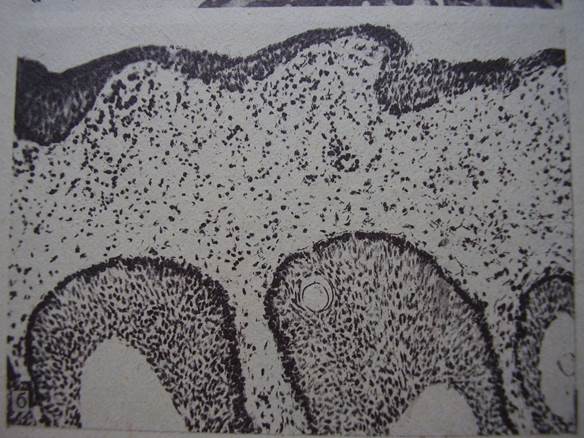
Fig. 3. A microphotograph from the bladder mucosa. Translation of the caption: «Carcinoma in situ». Magnification x100. [46]. No clear signs of neoplasia or dysplasia are visible in this image. There are somewhat hyperplastic von Brunn’s nests at the bottom.
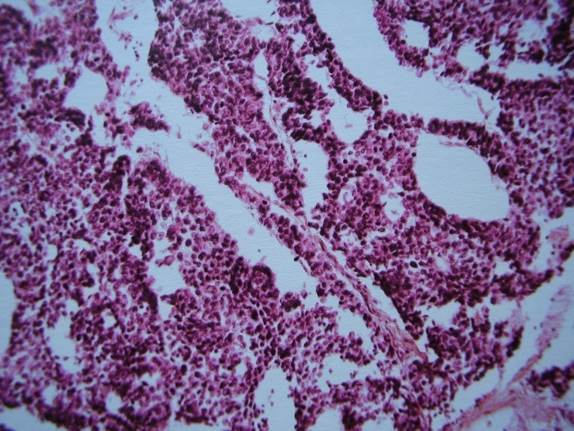
Fig. 4. Translation of the caption: «Moderately differentiated transitional cell carcinoma, grade III». Magnification is not indicated [47]. Moderately differentiated carcinoma is usually designated as Grade II. The section may be tangential. There is no marked atypia, which is to await in the Grade III carcinoma of the bladder.
The results of the study [4] might appear inconclusive: 'These findings do not allow us to consider the immunohistochemical expression of ubiquitylation and sumoylation as valuable markers for discriminating the effects of long-term, low-dose IR exposure in cRCC (conventional renal cell carcinoma) carcinogenesis.' However, taking into account that the cancers diagnosed in Ukraine must have been on average more advanced than the controls from Spain, the results of this study suggest that ubiquitylation and sumoylation are not significantly associated with the neoplastic progression of renal cell carcinoma. On the contrary, the RET/PTC3 rearrangements can be associated with the patients’ age [50,51] and probably also with the progression of papillary TC [28,52]. In agreement with this hypothesis, an association was reported between RET/PTC3 and a more aggressive phenotype, a greater tumor size and a more advanced stage at diagnosis [51]. An association with the tumor progression can exist also for some markers of renal cell carcinoma, where significant differences between the Ukrainian and Spanish cohorts were found in the studies [3,15].
The above and previously published [26–32] arguments question in principle the cause-effect relationship between ionizing radiation and cancer incidence increase after the Chernobyl accident. In regard to the Chernobyl-related TC, such cause-effect relationship cannot be excluded, but the registered incidence increase was largely caused by factors other than radiation. It means that some studies searching for markers of radiation-related post-Chernobyl cancer, e.g. [53], were partly based on the unproven supposition that all or a majority of malignancies in the contaminated areas had been caused or influenced by ionizing radiation [27–31]. A concluding point is that results of some Chernobyl-related molecular-genetic studies should be re-evaluated, considering that many tumors detected after the accident due to the screening and improved diagnostics, or brought from non-contaminated areas, were relatively advanced, previously neglected cancers, sometimes misinterpreted as aggressive radiogenic tumors developing after a short latent period. It can be confirmed by the following citation: 'The tumors were randomly selected (successive cases) from the laboratories of Kiev and Valencia... [The cancers were] clearly more aggressive in the Ukrainian population in comparison with the Valencian cases' [53]. This phenomenon has an obvious explanation: on average earlier diagnostics of malignancies in Valencia. The conclusions of this paper might be useful for elaboration of the radiation safety norms [32].
Литература:
1. UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation). Sources and effects of ionizing radiation. Vol. I. Sources of ionizing radiation. Annex B. Exposures of the public and workers from various sources of radiation. Vol. II. Effects of ionizing radiation. Annex D: Health effects due to radiation from the Chernobyl accident. New York: United Nations, 2008.
2. Боровикова Н. М., Бурлак Г. Ф., Бережная Т. И., Варбанец А. Н., Ткаченко Н. В., Чуприна С. В. Формирование дозы облучения населения Киева после аварии на Чернобыльской АЭС // В сб. Итоги оценки медицинских последствий аварии на Чернобыльской АЭС. Тезисы докладов на научно-практической конференции. Киев, 1991, стр. 33–34.
3. Romanenko A., Morell-Quadreny L., Ramos D. et al. Extracellular matrix alterations in conventional renal cell carcinomas by tissue microarray profiling influenced by the persistent, long-term, low-dose ionizing radiation exposure in humans. Virchows Archiv 2006, V 448, p. 584–590.
4. Morell-Quadreny L., Romanenko A., Lopez-Guerrero J. A. et al. Alterations of ubiquitylation and sumoylation in conventional renal cell carcinomas after the Chernobyl accident: a comparison with Spanish cases. Virchows Archiv 2011, V 459, p. 307–313.
5. UNSCEAR. Sources and effects of ionizing radiation. Vol. I. Sources. Annex B. Exposures from natural radiation sources. Vol. II. Effects. Annex G: Biological effects at low radiation doses. Annex I: Epidemiological evaluation of radiationinduced cancer. New York: United Nations, 2000.
6. Ghiassi-nejad M., Mortazavi S. M., Cameron J. R. et al. Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health Physics 2002, V 82, p. 87–93.
7. Nair R. R., Rajan B., Akiba, S. et al. Background radiation and cancer incidence in Kerala, India-Karanagappally cohort study. Health Physics 2009, V 96, p. 55–66.
8. Tubiana M., Aurengo A., Averbeck D., Masse R. Recent reports on the effect of low doses of ionizing radiation and its dose-effect relationship. Radiation and Environmental Biophysics 2006, V 44, p. 245–251.
9. UNSCEAR. Summary of low-dose radiation effects on health. New York: United Nations, 2010
10. ICRP (International Commission on Radiological Protection). Lung cancer risk from radon and progeny and statement on radon. ICRP Publication 115. Annals of the ICRP 2010, V 40, N 1.
11. Mould R. F. The Chernobyl record. The definite history of Chernobyl catastrophe. Institute of Physics, Bristol & Philadelphia, 2000. — 402 p.
12. Mettler F. A. Jr., Huda W., Yoshizumi T. T., Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008, V 248, p. 254–263.
13. Romanenko A., Kakehashi A., Morimura K. et al. Urinary bladder carcinogenesis induced by chronic exposure to persistent low-dose ionizing radiation after Chernobyl accident. Carcinogenesis 2009, V 30, p. 1821–1831.
14. IAEA (International Atomic Energy Agency). Environmental Consequences of the Chernobyl Accident and their Remediation: Twenty Years of Experience. Report of the UN Chernobyl Forum Expert Group «Environment». Vienna: IAEA, 2006.
15. Romanenko A., Morell-Quadreny L., Nepomnyaschy V. et al. Pathology and proliferative activity of renal-cell carcinomas (RCCS) and renal oncocytomas in patients with different radiation exposure after the Chernobyl accident in Ukraine. International Journal of Cancer 2000, V 87, p. 880–883.
16. Romanenko A., Morimura K., Wei M. et al. DNA damage repair in bladder urothelium after the Chernobyl accident in Ukraine. Journal of Urology 2002, V 168, p. 973–977.
17. Romanenko A. M., Kinoshita, A., Wanibuchi H. et al. Involvement of ubiquitination and sumoylation in bladder lesions induced by persistent long-term low dose ionizing radiation in humans. Journal of Urology 2006, V 175, p. 739–743.
18. Takamura N., Yamashita S. Lessons from Chernobyl. Fukushima Journal of Medical Science 2011, V 57, p. 81–85.
19. Naing S., Collins B. J., Schneider A. B. Clinical behavior of radiation-induced thyroid cancer: factors related to recurrence. Thyroid 2009, V 19, p. 479–485.
20. Tuttle R. M., Vaisman F., Tronko M. D. Clinical presentation and clinical outcomes in Chernobyl-related paediatric thyroid cancers: what do we know now? What can we expect in the future? Clinical Oncology 2011, V 23, p. 268–275.
21. Feinmesser R., Lubin E., Segal K., Noyek A. Carcinoma of the thyroid in children — a review. Journal of Pediatric Endocrinology & Metabolism 1997, V 10, p. 561–568.
22. Gow K. W., Lensing S., Hill D. A. et al. Thyroid carcinoma presenting in childhood or after treatment of childhood malignancies: An institutional experience and review of the literature. Journal of Pediatric Surgery 2003, V 38, p. 1574–1580.
23. Jarzab B., Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens) 2007, V 6, p. 200–209.
24. Boltze C., Riecke A., Ruf C. G. et al. Sporadic and radiation-associated papillary thyroid cancers can be distinguished using routine immunohistochemistry. Oncology Reports 2009, V 22, p. 459–467.
25. Williams E. D., Abrosimov A., Bogdanova T. et al. Thyroid carcinoma after Chernobyl latent period, morphology and aggressiveness. British Journal of Cancer 2004, V 90, p. 2219–2224.
26. Яргин С. В. О преувеличении радиационных последствий аварии на Чернобыльской АЭС. Медицинская радиология и радиационная безопасность 2007, том 52, № 1, стр. 73–74
27. Яргин С. В. Преувеличенная оценка медицинских последствий повышения радиационного фона. Медицинская радиология и радиационная безопасность 2008, том 53, № 3, стр. 17–22
28. Яргин С. В. О завышенной оценке заболеваемости раком щитовидной железы среди лиц, подвергшихся в детском возрасте радиоактивному облучению при аварии на ЧАЭС. Медицинская радиология и радиационная безопасность 2010, том 55, № 2, стр. 65–69
29. Яргин С. В. К вопросу о завышенной оценке медицинских последствий аварии на ЧАЭС: причины и механизмы. Медицинская радиология и радиационная безопасность 2011, том 56, № 5, стр. 74–79.
30. Jargin S. V. Overestimation of Chernobyl consequences: biophysical aspects. Radiation and Environmental Biophysics 2009, V 48, 341–344.
31. Jargin S. V. Thyroid carcinoma in children and adolescents resulting from the Chernobyl accident: possible causes of the incidence increase overestimation. Ceskoslovenská Patologie 2009, V 45, p. 50–52.
32. Jargin S. V. Hormesis and radiation safety norms. Human & Experimental Toxicology 2012, V 31, p. 671–675.
33. Balonov M. I. The Chernobyl Forum: major findings and recommendations. Journal of Environmental Radioactivity 2007, V 96, p. 6–12.
34. IAEA. Chernobyl Forum, 2003–2005. Second revised version. Chernobyl’s legacy: health, environmental and socio-economic impacts and recommendations to the governments of Belarus, the Russian Federation and Ukraine. Vienna: IAEA, 2006.
35. WHO (World Health Organization). Health effects of the Chernobyl accident. Report of the UN Chernobyl Forum Expert Group «Health». B. Bennet, M. Repacholi, Z. Carr (Eds), Geneva: WHO, 2006.
36. Zablotska L. B., Ron E., Rozhko A. V. et al. Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. British Journal of Cancer 2011, V 104, p. 181–187.
37. Ivanov V. K., Tsyb A. F., Khait S. E. et al. Leukemia incidence in the Russian cohort of Chernobyl emergency workers. Radiation and Environmental Biophysics 2012, V 51, p. 143–149.
38. Stsjazhko V. A., Tsyb A. F., Tronko N. D. et al. Childhood thyroid cancer since accident at Chernobyl. BMJ 1995, V 310, p. 801.
39. IARC (International Agency for Research on Cancer). Ionizing radiation, Part 2. Some internally deposited radionuclides In IARC monographies on the evaluation of carcinogenic risks to humans. Lyon: IARC Press, 2001, V 78, p. 234.
40. Luster M., Lassmann M., Freudenberg L. S., Reiners C. Thyroid cancer in childhood: management strategy, including dosimetry and long-term results. Hormones (Athens) 2007, V 6, p. 269–278.
41. Parkin D. M., Kramárová E., Draper G. J. et al. International incidence of childhood cancer. IARC Scientific Publication 144. Lyon: IARC Press, 1999.
42. Demidchik Y. E., Saenko V. A., Yamashita S. Childhood thyroid cancer in Belarus, Russia, and Ukraine after Chernobyl and at present. Arquivos Brasileiros de Endocrinologia e Metabologia 2007, V 51, p. 748–762.
43. Лушников Е. Ф., Цыб А. Ф., Ямасита С. Рак щитовидной железы в России после Чернобыля. Москва: Медицина, 2006, стр. 36–59.
44. Di Cristofaro J., Vasko V., Savchenko V. et al. ret/PTC1 and ret/PTC3 in thyroid tumors from Chernobyl liquidators: comparison with sporadic tumors from Ukrainian and French patients. Endocrine-Related Cancer 2005, V 12, p. 173–183.
45. Romanenko A., Fukushima S. Prediction of urinary bladder cancer induction in Ukraine after the Chernobyl accident. XXIII International Congress of the International Academy of Pathology and 14th World Congress of Academic and Environmental Pathology. 15–20 October 2000, Nagoya, Japan. Pathology International 2000, V 50(Suppl), p. A70.
46. Романенко А. М., Клименко И. А., Юрах Г. Ю. Лейкоплакия мочевого пузыря. Архив патологии 1985, том 47, № 1, стр. 52–58.
47. Самсонов В. А. Опухоли почек и мочевыводящих путей // Краевский Н. А., Смольянников А. В., Саркисов Д. С. (ред.) Патологоанатомическая диагностика опухолей человека. Москва: Медицина, 1993, том 2, стр. 137–161.
48. Пальцев М. А., Аничков Н. М. Атлас патологии опухолей человека. Москва: Медицина, 2005, стр. 402.
49. Jargin S. V. Pathology in the former Soviet Union: scientific misсonduct and related phenomena. Dermatol Pract Concept 2011, V 1, N 1, Article 16. http://dx.doi.org/10.5826/dpc.0101a16
50. Trovisco,V., Soares P., Preto A. et al. Molecular genetics of papillary thyroid carcinoma: great expectations. Arquivos Brasileiros de Endocrinologia e Metabologia 2007, V 51, p. 643–653.
51. Romei C., Elisei R. RET/PTC Translocations and Clinico-Pathological Features in Human Papillary Thyroid Carcinoma. Frontiers in Endocrinology 2012, V 3, p. 54.
52. Jargin S. V. On the RET Rearrangements in Chernobyl-related thyroid cancer. Journal of thyroid research 2012, Article 373879.
53. Akulevich N. M., Saenko V. A., Rogounovitch T. I. et al. Polymorphisms of DNA damage response genes in radiation-related and sporadic papillary thyroid carcinoma. Endocrine-Related Cancer 2009, V 16, p. 491–503.
54. Romanenko A., Morell-Quadreny L., Ramos D. et al. Author reply to Jargin S. V. Over-estimation of radiation-induced malignancy after the Chernobyl accident. Virchows Archiv 2007, V 451, p. 107–108.







