1. Introduction
Pollution of heavy metals in water is a serious problem globally that needs to be solved. In Vietnam, the source of heavy metal emissions is mainly from industrial production facilities such as mechanics, machinery manufacturing, and electroplating, which have been and are polluting water sources due to direct discharge of waste into the environment without treatment. According to the 2008 national environment report, the content of heavy metals such as Cr 6+ , Zn 2+ , Pb 2+ , Cu 2+ in wastewater exceeds the permitted standards from 1.5 to 10 times [1].
Peanuts and rice are the main agricultural crops grown in our country. Therefore, peanut shells and rice husks are a readily available and inexpensive source of agricultural by-products. Every year, thousands of tons of rice husks and peanut shells are generated, but these agricultural by-products are mainly discarded or burned. Many studies have shown that they can be used to make adsorbent materials (VLHP) to treat water contaminated with heavy metals. Carbonization of peanut shells to treat and adsorb Cu 2+ from wastewater has been studied in comparison with GAC coal, resulting in 18 times higher efficiency or used to adsorb Cu 2+ , Zn 2+ with an efficiency of over 90 % [3]. In Vietnam, rice husk ash is used to treat Ni 2+ , Cd 2+ ions with a relatively high efficiency of 50–60 % [6].
2. Research methods
2.1. Materials and analysis methods
2.1.1. Raw materials — chemicals, equipment
Chemicals used in the experiment: CuSO 4 , Pb(NO 3 ) 2 , Murexit indicator, indicator ETOO, NaOH, HCl, EDTA, KNaC4H4O6.4H2O of Merk (Germany). Equipment used in the research: Jatest (VELP, JLT6, Italy) including 6 stirring systems operating in the same mode, drying cabinet (Blinder, USA), kiln (Lenton AF11/6B, UK). Meanwhile, the research materials are rice husks and peanut shells collected from Nam Dinh.
2.1.2. Preparation of adsorbents
Rice husks and peanuts are washed and dried at 80°C for 12 hour. The product, after drying, is soaked with 1M HCl for 120 minutes (for rice husk), and 20 grams of peanut shells, after grinding, are soaked with 1M HNO3 solution for 24 hours, then the rice husks and peanuts are washed with distilled water until the solution has a neutral environment. The rice husk, after washing, is dried at 80°C, then calcined at 550°C for 12 hours, obtaining the adsorbent material (VLHP T). And the peanut shells are calcined at 450°C for 12 hours to obtain the adsorbent material (VLHP L).
2.2. Description of the experiment
In each experiment, prepare 6 glass beakers of 1 liter, numbered from 1 to 6, add 100 ml of 0.01mM Cu2+ solution (6.4mg/l) or 0.01mM Pb 2+ solution (20.7mg/l). Then, in turn, add a specific mass of VLHP T, VLHP L to each beaker (to study the effect of pH and time, use 1g VL), adjust the pH with 0.1M HCl solution or 0.1M NaOH solution depending on the study. Stir the 6 beakers on a Jartest machine at 250 rpm for 60 minutes. Finally, filter the solution with filter paper and determine The remaining concentration of Cu 2+ and Pb 2+ in the solution (Cs) was determined by quantitative titration with EDTA. Each experiment was repeated twice to obtain the average result. The experiments were conducted at room temperature 25±2°C.
3.1. Properties of Adsorbent Materials
Two material samples were examined under a scanning electron microscope (JFM — 5410 LV, Japan). The images were taken at different magnifications: 5000x, 10000x, 20000x.
From Figure 1, it can be seen that on both surfaces of the adsorbent materials, many pores were formed, increasing the porosity of the material, making it easier to adsorb copper and lead ions. The modified rice husk ash material (VLHPT) has uniform capillaries, and the total surface area of the modified material increases many times. The reason is that during modification, strong acids like HNO 3 and HCl dissolve lignin or cellulose and increase the porosity of the adsorbent material [8]. In addition, after modification, functional groups such as -COOH, -OH, -NH 2 are formed, helping to increase the cation exchange capacity.
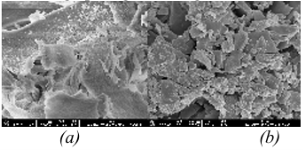
Fig. 1. 10000x magnification SEM image, (a) VLHP T (b) VLHP L
3.2 pH affects the adsorption capacity of 2 VLHPs
The research results show that pH affects the adsorption process of Cu 2+ , Pb 2+ of VLHP. The adsorption efficiency increases when the pH increases from 1 to 6. Because in a strong acid environment (pH≤3), the molecules of the adsorbent and the adsorbate are both positively charged, so the interaction force is an electrostatic repulsion force. Besides, the concentration of H+ ions is high, so in the mixture, there is a competitive reaction between H + ions and metal cations Cu 2+ , Pb 2+ in the adsorption process [2], resulting in a decrease in the adsorption efficiency of Cu 2+ , Pb 2+ ions. The adsorption efficiency of 2 VLHPs increases with increasing pH. However, at pH = 6, precipitation of Cu(OH) 2 , Pb(OH) 2 begins to occur in the solution, so pH = 5 is chosen as the appropriate pH for the adsorption of Pb 2+ , Cu 2+ by both modified VLHPs from rice husk ash and peanut shells.
3.3. The effect of VLHP mass on adsorption efficiency
The mass of 2 VLHPs was varied (2, 4, 6, 8, 10, 12, 20 mg/l) at pH=5. The results show that increasing the mass leads to an increase in the adsorption efficiency of the VLHPs because there are more VLHP particles in the same volume, so the contact surface between them and Cu 2+ , Pb 2+ increases, and the ability of metal ions to enter the capillaries of VLHP increases. The adsorption efficiency of Cu 2+ and Pb 2+ is highest when the initial VLHP mass is 20mg/l. Using VLHP T, the adsorption efficiency is 98.5 % and 97.7 % when using VLHP L. Meanwhile, the adsorption of Pb 2+ ions reaches the highest value of 99.2 % and 98.5 % when using VLHP T and VLHP L, respectively.
3.4 The effect of time on the adsorption capacity of VLHPs
The study shows that the mass of the material affects the adsorption efficiency of the adsorbent material. For VLHP T, as time increases, the adsorption efficiency also increases. As the stirring time increases, more Pb 2+ enters the capillaries of the VLHP, so the adsorption efficiency increases. Specifically, the efficiency increases rapidly to 95 % in the first 90 minutes and increases insignificantly in the next minutes of 120 minutes and 150 minutes (reaching 99.7 % and 99.3 %). Thus, VLHP T reaches adsorption equilibrium at 90 minutes. With VLHP L, similar to VLHP T, it reaches equilibrium at 90 minutes (adsorption efficiency of 94.8 %) and then gradually increases to 99.5 % at 150 minutes. Similar to the adsorption of Pb 2+ ions, both VLHPs reach equilibrium at 90 minutes with efficiencies of 92.9 % and 91.3 %, respectively, when adsorbed by VLHP T and VLHP L.
3.5. Langmuir isothermal adsorption equation
The study of establishing the Langmuir isotherm equation was conducted with the optimal adsorption pH of 5 and a duration of 90 minutes, using 2g of VLHP.
The results show that when the initial concentration of Cu 2+ and Pb 2+ solutions increases, the adsorption capacity of the material also gradually increases. The Langmuir equation, maximum adsorption capacity, and Langmuir constant K1 are presented in Table 1.
Table 1
Constant of Langmuir isothermic equation adsorption Pb 2+ , Cu 2+ of adsorbents
|
q m (mg/g) |
K L (l/mg) |
R 2 |
||
|
Pb 2+ |
VLHPT |
62.1 |
55,42 |
0,99 |
|
VLHPL |
48.62 |
22,3 |
0,997 |
|
|
Cu 2+ |
VLHPT |
50.2 |
22,51 |
0,989 |
|
VLHPL |
40.5 |
26,6 |
0,99 |
From Table 1, we have R 2 values of the Langmuir equations for Cu 2+ and Pb 2+ adsorption ranging from 0.989 to 0.99, so we can conclude that the adsorption of Cu 2+ and Pb 2+ ions follows the Langmuir isotherm equation. According to Table 1, VLHPT treats Cu 2+ and Pb 2+ better than VLHPL, with maximum adsorption capacities of 50 mg/g and 62.11 mg/g (VLHPT), respectively, compared to 40.49 mg/g and 47.62 mg/g when treated with VLHPL.
The results show that VLHPT and VLHPL in this study adsorb Pb 2+ and Cu 2+ well. For Cu 2+ , VLHPT (50 mg/g) and VLHPL (40.49 mg/g) are higher than the adsorbent materials synthesized from peanut shells [2] (0.3451 mg/g), barley husks [5] (4.64 mg/g), pine bark [4] (3.12 mg/g), and even rice husk treated with tartaric acid [9] (29 mg/g). For Pb 2+ ions, the adsorption capacity of VLHPT and VLHPL is also quite good, higher than modified straw [6] and barley husks [5] with values of 62.11 mg/g and 47.62 mg/g compared to 55.56 mg/g and 23.2 mg/g.
4. Conclude
This study has demonstrated that peanut shells and rice husks modified with HNO 3 and HCl acids and calcined at high temperatures have a good ability to adsorb Cu 2+ and Pb 2+ ions. The optimal conditions for the adsorption process of The optimal conditions for the adsorption process of Cu 2+ and Pb 2+ ions are as follows: pH = 5, equilibrium time of 90 minutes. The adsorption process follows the Langmuir equation. This study has shown a new direction for the reuse of agricultural by-products in treating environmental pollution.
References:
- General Department of Environment (2008). National Environmental Report 2008 — Vietnam Craft Village Environment (in Vietnamese).
- Abdel Salam, O. E., Reiad, N. A., & ElShafei, M. M. 2011. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. Journal of Advanced Research, 2(4), 297–303.
- Kermit Wilson, H. Y., Chung W. Seo, Wayne E. Marshall. (2006). Select metal adsorption by activated carbon made from peanut shells. Bioresource technology, 97(2266–2270).
- Najua, D. T., Luqman, C. A., Zawani, Z., & Suraya, A. R. (2008). Adsorption of copper from aqueous solution by Elais Guineensis kernel activated carbon. Journal of Engineering Science and Technology, 3(2), 180–189.
- Pehlivan, E., Altun, T., & Parlayıcı, S. (2009). Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. Journal of Hazardous Materials, 164(2), 982–986.
- Phạm Hoàng Giang, Đ. Q. H. 2016. Nghiên cứu xử lý kim loại nặng trong nước bằng phương pháp hấp phụ trên phụ phẩm nông nghiệp biến tính axit photphoric Tạp chí Khoa học ĐHQGHN: Các Khoa học Trái đất và Môi trường, 32.
- Riaz, M., Nadeem, R., Hanif, M. A., Ansari, T. M., & Rehman, K.U. 2009. Pb(II) biosorption from hazardous aqueous streams using Gossypiumhirsutum (Cotton) waste biomass. Journal of Hazardous Materials, 161(1), 88–94.
- Vieira M. G. A, Silva, C. N. Carneiro, A. A. Melo Filho. 2014. Adsorption of lead and copper ions from aqueous effluents on rice husk ash in dynamic system. Brazilian Journal of Chemical Engineering, 31(2), 519–529.
- Wong, K. K., Lee, C. K., Low, K. S., & Haron, M. J. 2003. Removal of Cu and Pbby tartaric acid modified rice husk from aqueous solutions. Chemosphere, 50(1), 23–28.







