Definitions
Pulmonary embolism (PE): luminal obstruction of one or more pulmonary arteries, typically due to blood thrombi from deep vein thrombosis (DVT)
Venous thromboembolism (VTE): an umbrella term that encompasses PE and DVT (see also «Hypercoagulable states»)
Recurrent VTE : VTE that recurs in a patient after the completion of the first 2 weeks of antithrombotic therapy [1].
Provoked VTE : VTE in an individual with ≥ 1 risk factor for VTE
Unprovoked VTE (idiopathic VTE): VTE in an individual without risk factors for VTE
Epidemiology: Venous thromboembolism (VTE), clinically presenting as DVT or PE, is globally the third most frequent acute cardiovascular syndrome behind myocardial infarction and stroke. In epidemiological studies, annual incidence rates for PE range from 39 to 115 per 100 000 population; for DVT, incidence rates range from 53 to 162 per 100 000 population.
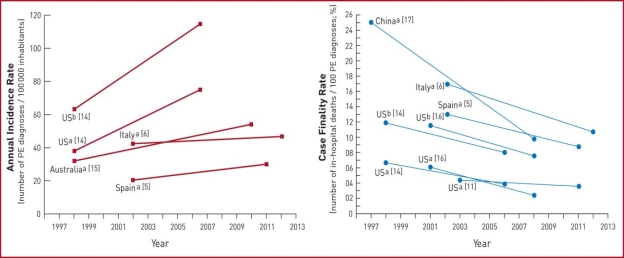

Fig. 1. summarizes the existing data on global trends in PE, highlighting increasing incidence rates in parallel with decreasing case fatality rates over an ∼15 year period
Trends in annual incidence rates (left panel) and case fatality rates (right panel) of pulmonary embolism around the world. Reproduced with permission from. PE: pulmonary embolism; US: United States. a PE listed as principal diagnosis. b Any listed code for PE was considered
Etiology
Nearly all pulmonary emboli arise from thrombi in the veins of the legs or pelvis (deep venous thrombosis). Risk of embolization is higher with thrombi that reach the popliteal vein or above. Thromboemboli can also originate in arm veins or central veins of the chest (caused by central venous catheters or resulting from thoracic outlet syndromes).
Pulmonary embolism can also arise from nonthrombotic sources (eg, embolism of air, amniotic fluid, fat, infected material, orthopedic cement, foreign body, tumor).
Risk factors for deep venous thrombosis and pulmonary embolism (see table Risk Factors for Deep Venous Thrombosis and Pulmonary Embolism) are similar in children and adults and include
– Conditions that impair venous return, including bed rest and confinement without walking
– Conditions that cause endothelial injury or dysfunction such as trauma or surgery
– Underlying hypercoagulable (thrombophilic) disorders such as cancer or primary clotting disorders
– COVID-19 appears to be a risk factor for deep venous thrombosis and pulmonary embolism due to a hypercoagulable state responsible for large-vessel thrombosis and thromboembolism.
Symptoms and signs of pulmonary embolism
Many pulmonary emboli are small, physiologically insignificant, and asymptomatic. Even when present, symptoms are nonspecific and vary in frequency and intensity, depending on the extent of pulmonary vascular occlusion and preexisting cardiopulmonary function.
Emboli often cause
– Acute dyspnea
– Pleuritic chest pain (when there is pulmonary infarction)
Dyspnea may be minimal at rest and can worsen during activity.
Less common symptoms include
– Cough (usually caused by comorbid disorders or by dilation of the pulmonary arteries)
– Hemoptysis (occasionally occurs when there is pulmonary infarction)
In older patients, the first symptom may be altered mental status. Massive pulmonary emboli may manifest with hypotension, tachycardia, light-headedness/presyncope, syncope, or cardiac arrest.
The most common signs of pulmonary embolism are
– Tachycardia
– Tachypnea
Less commonly, patients have hypotension.
Risk factors
Major risk factors
– Recent surgery (abdominal/pelvic surgery, lower-limb orthopaedic surgery, post-operative intensive care)
– Pregnancy and, in particular, six weeks post partum
– Lower-limb fracture/varicose veins
– Active malignancy (particularly abdominal/pelvic or advanced/metastatic cancer)
– Reduced mobility (for example, during hospitalisation or while in institutional care)
– Deep vein thrombosis (DVT) — or previous DVT or pulmonary embolism
Minor risk factors
– Cardiovascular conditions (for example, congenital heart disease, heart failure, hypertension)
– Oestrogen (oral contraceptive pill, hormone replacement therapy)
– Miscellaneous (chronic obstructive pulmonary disease, occult malignancy, thrombotic disorders, obesity, long-distance travel)
– Aged >60 years

Diagnosis
The methods of diagnosing PE applied to specific patients depend primarily on determining the probability of the disease, the severity of the patient's condition and the capabilities of medical institutions.
The following algorithm is used to diagnose PE:
- Assessment of clinical probability (pre-test probability).
- Determination of the D-dimer level (taking into account age-adjusted thresholds and the level of clinical probability of PE).
- Computed tomography of the pulmonary artery with contrast enhancement.
- Lung scintigraphy is a study of pulmonary blood flow, in which a small amount of a radioactive substance is injected into the body, after which the process of distribution of this substance in organs and tissues is visualized using a gamma camera.
- Angiopulmonography is an invasive study of pulmonary circulation performed by introducing a radiopaque substance into the pulmonary arteries.
- Magnetic resonenace imaging.
- Echocardiography (bedside if there is a high probability of PE). 8. Compression ultrasound examination of veins.
– Assessment of clinical probability
When assessing the probability of PE, the following factors are taken into account: surgery or fracture in the previous month, malignant tumor, age over 65 years, hemoptysis, pain in the lower limb on the one hand, high heart rate.
– D-dimer in PE
The method of determining the D-dimer has proven its high importance in case of suspected PE. However, the test is not absolutely specific, since increased results are also found in the absence of thrombosis, for example, in pregnant women, the elderly, atrial fibrillation, and malignant neoplasms. Therefore, this study is not shown to patients with a high probability of disease. However, with a low probability, the test is informative enough to exclude thrombosis in the vascular bed.
In cases of suspicion and when PE is proven, additional laboratory tests include cardiac markers:
- troponin level (increases with ischemia more often in the right, but sometimes in the left ventricle of the heart);
- H-FABP, a cardiac fatty acid binding protein, provides additional prognostic information in acute pulmonary embolism;
- the level of natridiuretic peptide (BNP) and pro-BNP — increases with dysfunction of the right ventricle or PE.
– Computed tomography
Computed tomography of the chest with vascular contrast is a highly proven method for diagnosing
pulmonary embolism. It allows you to visualize both large and small branches of the pulmonary artery.
If it is impossible to perform chest CT (pregnancy, intolerance to iodine-containing contrast agents, etc.), it is possible to perform planar ventilation perfusion (V/Q) lung scintigraphy. This method can be recommended for many categories of patients, but today it remains inaccessible. — Unfortunately, not all clinics are equipped with isotope and angiographic laboratories. But the implementation of screening techniques during the initial treatment of the patient — ECG, chest X—ray, ultrasound of the heart, ultrasound of the veins of the lower extremities — allows the patient to be referred for MSCT (multi-section spiral computed tomography) and further examination.
– ECG for PE
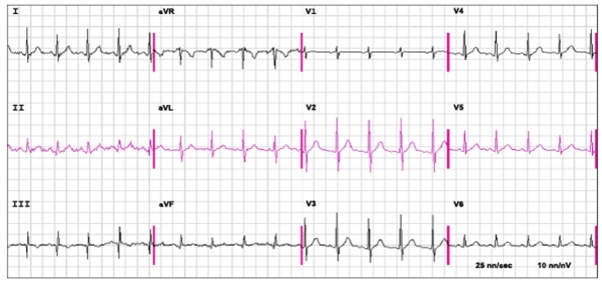
Fig. 2. The ECG shows sinus tachycardia at a rate of 110 beats/minute, an S1Q3T3 and R = S in V1 in a patient with proven acute pulmonary embolism
In the first place in terms of its diagnostic significance among instrumental examination methods is electrocardiography, which should be performed by all patients. Pathological changes in the ECG — acute overload of the right atrium and ventricle, complex rhythm disturbances, signs of coronary blood flow insufficiency — allow us to suspect the disease and choose the right tactics, determining the severity of the prognosis.
– Ultrasound with PE
To determine deep vein thrombosis, ultrasound of the veins of the lower extremities has high sensitivity and specificity, which can be performed at four points for screening: inguinal and popliteal areas on both sides. Increasing the study area increases the diagnostic value of the method.
Table 1
Imaging tests for diagnosis of pulmonary embolism
|
Strengths Weaknesses/limitations Radiation issues a |
|||
|
CTPA |
– Readily available around the clock in most centres – Excellent accuracy – Strong validation in prospective manage- – ment outcome studies – Low rate of inconclusive results (3–5 %) – May provide alternative diagnosis if PE – excluded – Short acquisition time |
– Radiation exposure – Exposure to iodine contrast: – limited use in iodine allergy and hyperthyroidism – risks in pregnant and breastfeeding women – contraindicated in severe renal failure – Tendency to overuse because of easy – accessibility – Clinical relevance of CTPA diagnosis of – subsegmental PE unknown |
– Radiation effective dose 3–10 mSvb – Significant radiation exposure to young female breast tissue |
|
Planar V/Q scan |
– Almost no contraindications – Relatively inexpensive – Strong validation in prospective manage- – ment outcome studies |
– Not readily available in all centres – Interobserver variability in interpretation – Results reported as likelihood ratios – Inconclusive in 50 % of cases – Cannot provide alternative diagnosis if PE – excluded |
– Lower radiation than CTPA, effective dose –2 mSvb |
|
V/Q SPECT |
– Almost no contraindications – • Lowest rate of non-diagnostic tests (<3 %) • High accuracy according to available data • Binary interpretation (‘PE’ vs. ‘no PE’) |
– Variability of techniques – Variability of diagnostic criteria – Cannot provide alternative diagnosis if PE – excluded – No validation in prospective management – outcome studies |
– Lower radiation than CTPA, effective dose –2 mSvb |
|
Pulmonary • Historical gold standard • Invasive procedure • Highest radiation, effective angiography • Not readily available in all centres dose 1020 mSvb |
|||
CTPA = computed tomographic pulmonary angiography; mGy = milligray; mSv = millisieverts; PE = pulmonary embolism; SPECT = single-photon emission computed tomogra- phy; V/Q = ventilation/perfusion (lung scintigraphy).
Complication of pulmonary embolism
– Acute pulmonary embolism can cause cardiac arrest and sudden death. With gradual development, chronic thromboembolic pulmonary hypertension occurs, progressive right ventricular circulatory failure.
– Chronic thromboembolic pulmonary hypertension (CTHEPH) is a form of the disease in which thrombotic obstruction of the small and medium branches of the pulmonary artery occurs, resulting in increased pressure in the pulmonary artery and increased load on the right parts of the heart (atrium and ventricle)
– HTEPH is a unique form of the disease because it can be potentially curable by surgical and therapeutic methods. The diagnosis is established on the basis of pulmonary artery catheterization data: increased pulmonary artery pressure above 25 mmHg, increased pulmonary vascular resistance above 2 units of Wood, detection of emboli in the pulmonary arteries against the background of prolonged anticoagulant therapy for more than 3–5 months.
– A severe complication of HTEPH is progressive right ventricular circulatory failure. Characteristic is weakness, palpitations, decreased load tolerance, the appearance of edema on the lower extremities, accumulation of fluid in the abdominal cavity (ascites), chest (hydrothorax), cardiac sac (hydropericardium). At the same time, there is no shortness of breath in a horizontal position, there is no stagnation of blood in the lungs. It is often with such symptoms that the patient first comes to a cardiologist. There is no data on other causes of the disease. Prolonged decompensation of blood circulation causes dystrophy of internal organs, protein starvation, and weight loss. The prognosis is most often unfavorable, temporary stabilization of the condition is possible against the background of drug therapy, but the reserves of the heart are quickly exhausted, edema progresses, life expectancy rarely exceeds 2 years.
Treatment
– Supportive therapy
– Anticoagulation
– Inferior vena cava filter placement (rarely, in selected patients)
– Rapid clot burden reduction via thrombolysis or embolectomy (in selected patients) supportive treatments, such as oxygen or analgesia, may be required. People are often admitted to hospital in the early stages of treatment and tend to remain under inpatient care until the INR has reached therapeutic levels (if warfarin is used).
Initial anticoagulation followed by maintenance anticoagulation is indicated for patients with acute pulmonary embolism to prevent further embolization as well as new clot formation. Anticoagulant therapy for acute PE should be started whenever PE is strongly suspected, as long as the risk of bleeding is deemed low. Otherwise, anticoagulation should be started as soon as the diagnosis is made. Time to therapeutic anticoagulation has been found to have an impact on mortality. Patients who attain therapeutic anticoagulation within 24 hours have been found to have a significant improvement in hospital mortality as well as 30 day mortality.
The likelihood of benefit versus harm in treating emboli in smaller, subsegmental vessels (particularly asymptomatic and incidentally discovered emboli) is unknown, and it is possible that for certain patients harm may outweigh benefit. Still, treatment is recommended for the vast majority of patients.
The primary complication of anticoagulation therapy is bleeding, and patients should be closely observed for bleeding during hospitalization.
Initial anticoagulation
Initial anticoagulation choices for acute PE include
– Intravenous unfractionated heparin
– Subcutaneous low molecular weight heparin
– Factor Xa inhibitors (oral apixaban, edoxaban, or rivaroxaban, or subcutaneous fondaparinux)
– Direct thrombin inhibitors (IV argatroban, oral dabigatran) for patients with heparin-induced thrombocytopenia
Intravenous unfractionated heparin has a short half-life (useful when the potential for bleeding is deemed higher than usual) and is reversible with protamine. An initial bolus of unfractionated heparin is given, followed by an infusion of heparindosed by protocol to achieve an activated partial thromboplastin time (PTT) 1.5 to 2.5 times that of normal control. Therefore, unfractionated heparin requires ongoing hospitalization to administer. Further, the pharmacokinetics of unfractionated heparin are relatively unpredictable, resulting in frequent periods of over-anticoagulation and under-anticoagulation and necessitating frequent dose adjustments. Some clinicians prefer this IV unfractionated heparin regimen when thrombolytic therapy is given or contemplated or when patients are at risk of bleeding because if bleeding occurs, the short half-life means that anticoagulation is quickly reversed after the infusion is stopped.
Subcutaneous low molecular weight heparin has several advantages over unfractionated heparin including
– Superior bioavailability
– Weight-based dosing results in a more predictable anticoagulation effect than does weight-based dosing of unfractionated heparin, which allows for quicker time to therapeutic coverage
– Ease of administration (can be given subcutaneously once or twice a day)
– Decreased incidence of bleeding
– Potentially better outcomes
– The potential for patients to self-inject (thereby allowing earlier discharge from the hospital)
– Lower risk of heparin-induced thrombocytopenia compared with standard, unfractionated heparin
Low molecular weight heparins that can be used include dalteparin, enoxaparin, and tinzaparin.
In patients with renal insufficiency, dose reductions are needed, and subsequent verification of appropriate dosing should be done by checking serum factor Xa levels (target: 0.5 to 1.2 IU/mL measured at 3 to 4 hours after the fourth dose). Low molecular weight heparins are generally contraindicated in patients with severe renal insufficiency (creatinine clearance < 30 mL/minute [0.5mL/second/m2]). Low molecular weight heparins are partially reversible with protamine.
Adverse effects of all heparins include
– Bleeding
– Thrombocytopenia (including heparin-induced thrombocytopenia with the potential for thromboembolism)
– Urticaria
– Anaphylaxis (rare)
Bleeding caused by over-heparinization with unfractionated heparin can be treated with a protamine infusion. Over-heparinization with a low molecular weight heparin can also be treated with protamine.
Fondaparinux is a factor Xa antagonist given subcutaneously. It can be used in acute DVT and acute PE instead of heparin or low molecular weight heparin. Outcomes appear to be similar to those of unfractionated heparin. Advantages include once or twice a day fixed-dose administration, no need for monitoring of the degree of anticoagulation, and lower risk of thrombocytopenia. The medication is contraindicated if creatinine clearance is < 30 mL/minute (0.5mL/second/m2).
Maintenance anticoagulation
Maintenance anticoagulation is indicated to reduce the risk of clot extension or embolization and to reduce the risk of new clot formation. Medication choices for maintenance anticoagulation include
– Oral vitamin K antagonist (VKA) (warfarin in the United States)
– Oral factor Xa inhibitors (apixaban, rivaroxaban, edoxaban)
– Oral direct thrombin inhibitor (dabigatran)
– Rarely subcutaneous low molecular weight heparin or subcutaneous fondaparinux
Warfarin is an effective long-term oral anticoagulant option that has been used for decades, but it is inconvenient for a number of reasons. In most patients, warfarin is started on the same day as heparin (or fondaparinux) therapy used for initial anticoagulation. Heparin (or fondaparinux) therapy should be overlapped with warfarin therapy for a minimum of 5 days anduntil the international normalized ratio (INR) has been within the therapeutic range (2.0 to 3.0) for at least 24 hour.
Table 2
Treatment of right ventricular failure in acute high-risk pulmonary embolism (European Society)
|
Strategy |
Properties and use |
Caveats |
|
Volume optimization |
||
|
Cautious volume loading, saline, or Ringer's lactate, ≤500 mL over 15–30 min |
Consider in patients with normal–low central venous pressure (due, for example, to concomitant hypovolaemia) |
Volume loading can over-distend the RV, worsen ventricular interdependence, and reduce CO [239] |
|
Vasopressors and inotropes |
||
|
Norepinephrine, 0.2–1.0 µg/kg/min a [240] |
Increases RV inotropy and systemic BP, promotes positive ventricular interactions, and restores coronary perfusion gradient |
Excessive vasoconstriction may worsen tissue perfusion |
|
Dobutamine, 2–20 µg/kg/min [241] |
Increases RV inotropy, lowers filling pressures |
May aggravate arterial hypotension if used alone, without a vasopressor; may trigger or aggravate arrhythmias |
|
Mechanical circulatory support |
||
|
Veno–arterial ECMO/extracorporeal life support [251, 252, 258] |
Rapid short-term support combined with oxygenator |
Complications with use over longer periods (>5–10 days), including bleeding and infections; no clinical benefit unless combined with surgical embolectomy; requires an experienced team |
CO: cardiac output; BP: blood pressure; ECMO: extracorporeal membrane oxygenation; RV: right ventricle/ventricular. a Epinephrine is used in cardiac arrest.
Reperfusion treatment
Thrombolytic therapy leads to faster improvements in pulmonary obstruction, PAP, and PVR in patients with PE, compared with UFH alone; these improvements are accompanied by a reduction in RV dilation on echocardiography.
Table3
Thrombolytic regimens, doses, and contraindications (European Society)
|
Molecule Regimen Contraindications to fibrinolysis |
||
|
rtPA |
100 mg over 2 h |
Absolute History of haemorrhagic stroke or stroke of unknown origin Ischaemic stroke in previous 6 months Central nervous system neoplasm Major trauma, surgery, or head injury in previous 3 weeks Bleeding diathesis Active bleeding Relative Transient ischaemic attack in previous 6 months Oral anticoagulation Pregnancy or first post-partum week Non-compressible puncture sites Traumatic resuscitation Refractory hypertension (systolic BP >180 mmHg) Advanced liver disease Infective endocarditis Active peptic ulcer |
|
0.6 mg/kg over 15 min (maximum dose 50 mg)a |
||
|
Streptokinase 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h
Accelerated regimen: 1.5 million IU over 2 h
|
||
|
Urokinase |
4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h |
|
|
Accelerated regimen: 3 million IU over 2 h |
||
BP = blood pressure; IU = international units; rtPA, recombinant tissue-type plasminogen activator. This is the accelerated regimen for rtPA in pulmonary embolism; it is not officially approved, but it is sometimes used in extreme haemodynamic instability such as cardiac arrest.
Prognostic assessment strategy
The classification of PE severity and the risk of early (in-hospital or 30 day) death is summarized in Table 8
Table4
Classification of pulmonary embolism severity and the risk of early (in-hospital or 30 day) death
|
Early mortality risk |
Indicators of risk |
||||
|
Haemodynamic instabilitya |
Clinical parameters of PE severity and/ or comorbidity: PESI class III–V or sPESI ≥1 |
RV dysfunction on TTE or CTPAb |
Elevated cardiac troponin levelsc |
||
|
High |
+ |
(+)d |
+ |
(+) |
|
|
Intermediate |
Intermediate–high |
- |
+e |
+ |
+ |
|
Intermediate–low |
- |
+e |
One (or none) positive |
||
|
Low |
- |
- |
- |
Assesment optional; if assessed, negative |
|
BP = blood pressure; CTPA = computed tomography pulmonary angiography; H-FABP = heart-type fatty acid-binding protein; NT-proBNP = N-terminal pro B-type natriuretic peptide; PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; RV = right ventricular; sPESI = simplified Pulmonary Embolism Severity Index; TTE = trans- thoracic echocardiogram.
There are several markers used for risk stratification and these are also independent predictors of adverse outcomes. These include hypotension, cardiogenic shock, syncope, evidence of right heart dysfunction, and elevated cardiac enzymes. Some ECG changes including S1Q3T3 also correlate with a worse short-term prognosis.
References:
- Моисеева О. М. Тромбоэмболия легочной артерии алгоритм диагностики и лечения. М. ГЭОТАР -Медиа, 2016.
- Рекомендации по диагностике и лечению тромбоэмболии легочной артерии. Рациональная фармакотерапия в кардиологии, No 1–2, 2009. — С. 96–111.
- Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, Bonnet D, Schulze-Neick I, Barst RJ. Clinical features of paediatric pulmonary hypertension:a registry study. Lancet 2012; 379(9815):537–546.
- Cummings KW, Bhalla S. Multidetector computed tomographic pulmonary angiography: beyond acute pulmonary embolism. Radiol Clin North Am 2010; 48(1):51–65.
- Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G,Wang C, Group C-S. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013; 369:319–29.
- Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous Thromboembolism. Am J Prev Med. 2010; 38(4): pp. S495–S501.
- Prandoni P, Lensing AW, Prins MH, Ciammaichella M, Perlati M, Mumoli N,. Bucherini E, Visona A, Bova C, Imberti D, Campostrini S, Barbar S; PESIT. Investigators. Prevalence of pulmonary embolism among patients hospitalized for. syncope. N Engl J Med 2016;375:15241531..
- Stein PD, Henry JW. Clinical characteristics of patients with acute pulmonary embo- lism stratified according to their presenting syndromes. Chest 1997;112:974–979.
- Barco S, Ende-Verhaar YM, Becattini C, Jimenez D, Lankeit M, Huisman MV, Konstantinides SV, Klok FA. Differential impact of syncope on the prognosis of patients with acute pulmonary embolism: a systematic review and meta-analysis..Eur Heart J 2018;39:4186–4195.
- Francalanci I, Comeglio P, Liotta AA, Cellai AP, Fedi S, Parretti E, Mello G, Prisco D, Abbate R. D-dimer concentrations during normal pregnancy, as meas- ured by ELISA. Thromb Res 1995;78:399–405.
- White RH. The epidemiology of venous thromboembolism. Circulation.. 2003;107:I4—I8.
- Jiménez D, Yusen RD, Otero R, Uresandi F, Nauffal D, Laserna E, et al. (July 2007). «Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy». Chest. 132 (1): 24–30. doi:10.1378/chest.06–2921. PMID 17625081







