Investigated the bioconversion of 2-ethylpyridine by the fungus Beauveria bassiana ATCC 7159. In the result of researches was obtained the hydroxylated derivative of the initial substrate. The yield of the product was observed as 60 %.
Keywords: bioconversion, fungi, Beauveria bassiana, 2-ethylpyridine
Introduction
Microbial hydroxylation is very important for obtaining new compounds used in organic synthesis [1–12]. Known not so many strains of microorganisms are able to carry out the hydroxylation of pyridines [13,14].
Some fungi are capable of hydroxylating of alkyl substituents of pyridines without affecting the heterocyclic ring. This allows you to get the appropriate hydroxyalkyl pyridines of interest to pharmacology with preparative yield [15].
The aim of this work was to study of the microbial hydroxylation of the methylene group of the alkyl substituent of 2-ethylpyridine. The product of such hydroxylation has optical activity and can be used in the synthesis of various valued medicines.
Materials and Methods
We used the strain of fungus Beauveria bassiana ATCC 7159 from the American Type Culture Collection.
The process of hydroxylation was carried out in a buffer solution of pH 6.0, for 48 hours by suspension of non-reproducing cells which previously grown up to stationary phase in the medium of the following composition (g/L): glucose — 20.0; corn steep liquor — 10.0; peptone — 5.0; KH2PO4–5.0; and deionized water, 1000 ml; the pH was adjusted to 5.0. 2-ethylpyridine (I) was added to the buffer mixture in an amount of 100 mg/L. The products of transformation were extracted from the culture medium by extraction with chloroform and separated on a column in the solvent system — hexane-ethyl acetate-methanol (5:5:1). For column chromatography was used silica gel — Kieselgel 0.200±0.036 (Merck AG, Germany). Thin-layer chromatography was performed on plates Silica gel 60 F254 (Merck KgaA, Darmstadt, Germany).
Electron ionization (EI) mass spectrometry was performed at an electron energy of 80 eV on the instrument Varian MAT-112 and on the instrument MX 1321A with the electron energy of 70 eV.
1H nuclear magnetic resonance (NMR) spectral analyses were performed at 60 MHz Tesla BS-467 (Czech Republic) NMR spectrometer operating at 28°C. Compounds were dissolved in CCl4.
Optical rotations were measured on a polarimeter Chemapol IV (Rudolph, USA)
Results and discussion
It has been found that B. bassiana ATCC 7159 transformed 2-ethylpyridine (I) into (-)-2-(1-hydroxyethyl)pyridine (II), in a yield of 60 % (Fig. 1). In extracts was detected product II (Rf= 0,44) moreover there was detected presence of starting material I.
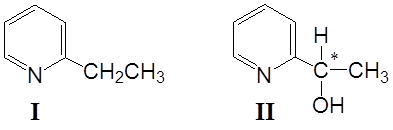
Fig. 1. Structures of 2-ethylpyridine (I) and of 2- (1-hydroxyethyl)pyridine (II)
The EI mass spectrum of compound II (m/z, I %): 123 [М+] (5), 122 [М-Н]+ (3), 108 [М-СН3]+ (100), 106 [М-ОH]+ (47), 105 [М-H2О]+ (14), 80 [М-CH3-СО]+ (47), 79 [М-CH3-СHО]+ (70), 78 [М-CH3-СHОH]+ (47), 53 [М-CH3-СО-HCN]+ (35).
To confirm the structure of the compound II was investigated its 1H NMR spectrum (CCl4) δ 1,36 d (3Н, СН3, 7,0), 4,53 s (1Н, ОН), 4,73 q (1Н, СН, 7,0), 6,8–7,8 m (3Н, β, β', γ-CH), 8,33 d (1Н, α-СН, 5,0).
As a substrate for the hydroxylation was selected 2-ethylpyridine, that is of practical interest.
So if the alkyl substituent of that compound has a methylene group, there can be expected the hydroxylation and the formation of an optically active alcohol.
The desired product (-)-2-(1-hydroxyethyl)pyridine was obtained for 48 hours and was isolated on a colomn with a yield of 60 %, [α]D20 -56,7º (c 2.2, CH3OH).
Previously, it was described similar oxidation of ethylbenzene with yields from 3 to 60 %. Thus, in some cases, also was observed formation of optically active secondary alcohols (enantioselectivity with 5 to 97 %) [16].
Our proposed method for producing an optically active alcohol II has a great value, since some of its levorotatory enantiomers have the medicinal properties [17]. The fragment of pyridine is part of many drugs [18], including antimalarial [19].
Known chemical methods for the preparation of isomeric (-)-(1-hydroxyethyl)pyridines are complicated, multistage, require the use of expensive and aggressive reagents, making those technologies low.
Our proposed process has great advantages over known chemical methods for producing (-)-(1-hydroxyethyl)pyridines.
References:
1. Parshikov I. A., Sutherland J. B. Microbial transformations of antimicrobial quinolones and related drugs. // J. Ind. Microbiol. Biotechnol. 2012. V.39. N 12. P. 1731–1740. doi: 10.1007/s10295–012–1194-x
2. Parshikov I. A., Silva E. O., Furtado N. A. J. C. Transformation of saturated nitrogen-containing heterocyclic compounds by microorganisms. // Appl. Microbiol. Biotecnol. 2014. V.98. N 4. P.1497–1506. doi: 10.1007/s00253–013–5429–1
3. Parshikov I. A., Modyanova L. V., Dovgilevich E. V., Terentyev P. B., Vorobyeva L. I., Grishina G. V. Microbial transformations of nitrogen heterocycles. III. Microbial synthesis of 1-benzoylpiperidine and 1-benzoylpyrrolidine hydroxy derivatives. // Chem. Heterocycl. Compd. 1992. V.28, N 2. P.159–162. doi: 10.1007/BF00473936
4. Parshikov I. A., Terent'ev P. B., Piskunkova N. F., Gracheva R. A., Bulakhov G. A. Microbial Transformation of 4-Phenylpyrrolidone-2 Derivatives by Micellar Fungi. // Cheminform. 2010. V. 29. N 1. doi: 10.1002/chin.199801032
5. Modyanova L. V., Duduchava M. R., Piskunkova N. F., Grishina G. V., Terentyev P. B., Parshikov I. A. Microbial transformations of piperideine and pyridine derivatives. // Chem. Heterocycl. Compd. 1999. V.35. N 5. P. 580–586. doi: 10.1007/BF02324642
6. Williamson J. S., Parshikov I. A.,Avery M. A. Biotransformations of Artemisinin. in: Recent Progress in Medicinal Plants, (Phytochemistry and Pharmacology). 2007. V. 17. P.115–138. doi: 10.17686/sced_rusnauka_2007–1129
7. Parshikov I. A. Microbial conversions of terpenoids. 2015. M.: Editus, 100 p. doi: 10.17686/sced_rusnauka_2015–1130
8. Dovgilevich E. V., Parshikov I. A., Modyanova L. V., Terent'ev P. B., Bulakhov G. A. A novel microbial transformation of gamma-carboline derivative 3,6-dimethyl-9- [2-(2-methylpyrid-5-yl)ethyl]-1,2,3,4-tetrahydro-gamma-carboline. // Mendeleev Commun. 1991. N 2. P.42–43. doi: 10.1070/MC1991v001n02ABEH000024
9. Terent'ev P. B., Parshikov I. A., Grishina G. V., Piskunkova N. F., Chumakov T. I., Bulakhov G. A. Hydroxylation of the Multiple Bond in 1-Benzyl-3-methyl-Δ3-piperideine by Micellar Fungi. // Cheminform. 2010. V.29. N 1. doi: 10.1002/chin.199801033
10. Parshikov I. A., Freeman J. P., Williams A. J., Moody J. D., Sutherland J. B. Biotransformation of N-acetylphenothiazine by fungi. // Appl. Microbiol. Biotechnol. 1999. V. 52. P.553–557. doi: 10.1007/s002530051559
11. Williams A. J., Parshikov I. A., Moody J. D., Heinze T. M., Sutherland J. B. Fungal transformation of the antimicrobial agent during growth on poultry-litter materials. // J. Appl. Poultry Res. 2004. V.13. N 2. P. 235–240. doi: 10.1093/japr/13.2.235
12. Parshikov I. A., Terentyev P. B., Modyanova L. V., Duduchava M. R., Dovgilevich E. V., Butakoff K. A. Microbial transformation of 9-amino-1,2,3,4,5,6,7,8-octahydroacridine. // Chem. Heterocycl. Compd. 1994. V.30. N 5. P.627–628. doi: 10.1007/BF01169849
13. Parshikov I. A. Microbial conversions of nitrogenous heterocycles. 2015. M.: Editus, 130 p.
14. Khasaeva F. M., Zakharchuk L. M., Netrusov A. I., Parshikov I. A. Biodegradation of pyridine by Arthrobacter sp. // Natural Science. In: Young Scientist USA. 2014. V.1, P.50–52. doi: 10.17686/sced_rusnauka_2014–1127
15. Modyanova L. V., Vorobyeva L. I., Shibilkina O. K., Dovgilevich E. V., Terentyev P. B., Kost A. N. Microbial transformation of nitrogen-containing heterocyclic compounds. I. Hydroxylation of isomeric methyl- and dimethylpyridines by microscopic fungi. // Biotekhnologiya. 1990. N 3. P.24–27
16. Holland H. L., Bergen E. F., Cherchaian P. C., Khan S. H., Munoz B., Ninniss R. W., Richards D. Side chain hydroxylation of aromatic hydrocarbons by fungi. 1. Products and stereochemistry. // Can. J. Chem. 1987. V.65. P.502–507. doi: 10.1139/v87–087
17. Tilford Ch.H., Shelton R. S., Van Campen M. G. Histamine antagonists. Basically substitued pyridine derivates. // J. Am. Chem. Soc. 1948. V.70. P.4001–4006. doi: 10.1021/ja01192a010
18. Wang F., Langley R., Gulten G., Dover L. G., Besra G. S., Jacobs W. R., Sacchettini J. C. Mechanism of thioamide drug action against tuberculosis and leprosy. // J. Exp. Med. 2007. V.204. P.73–78. doi: 10.1084/jem.20062100
19. Schleiferböck S., Scheurer C., Ihara M., Itoh I., Bathurst I., Burrows J. N., Fantauzzi P., Lotharius J., Charman S. A., Morizzi J., Shackleford D. M., White K. L., Brun R., Wittlin S. In vitro and in vivo characterization of the antimalarial lead compound SSJ-183 in Plasmodium models. // Drug. Des. Devel. Ther. 2013. N 7.P.1377–84. doi: 10.2147/DDDT.S51298







